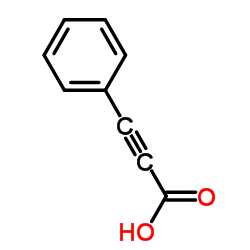
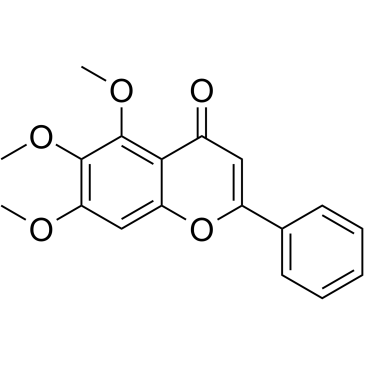
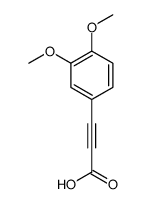
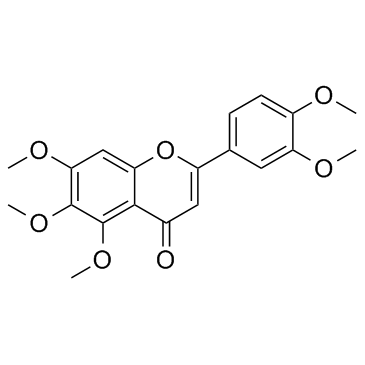
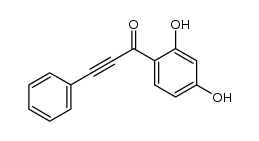
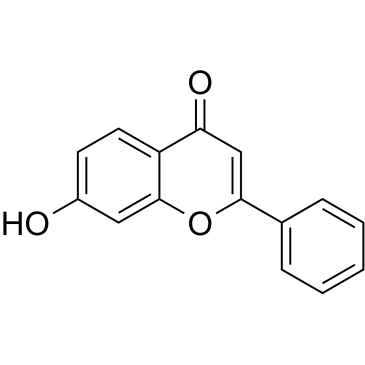
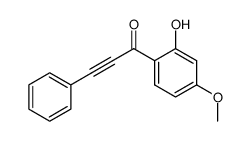
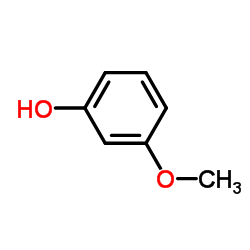
|
~63% |
|
~26% |
|
~96% |
|
~78% |
|
~% |
|
~% |
|
~4%
Detail
|