|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~37% |
|
~% |
|
~81% |
|
~% |
|
~% |
|
~% |
|
~80% |
|
~% |
|
~% |
|
~% |
|
~97% |
|
~% |
|
~% |
|
~% |
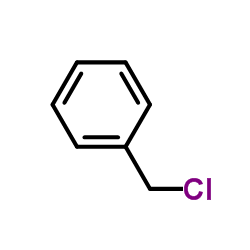
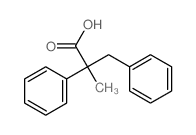
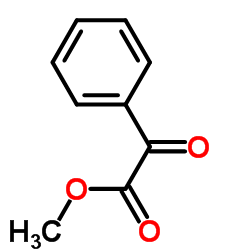
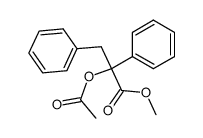
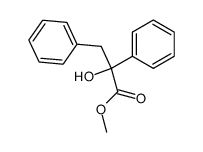
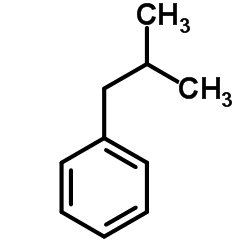
![diethyl 2-methyl-2-[4-(2-methylpropyl)phenyl]propanedioate Structure](https://image.chemsrc.com/caspic/297/62707-18-0.png)
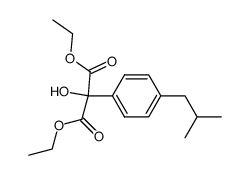

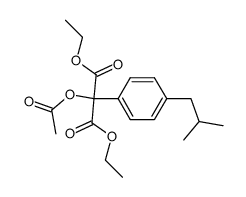

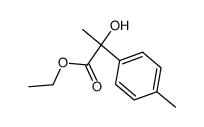
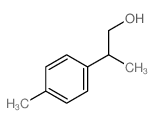
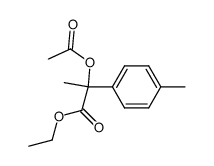
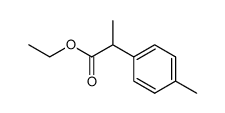
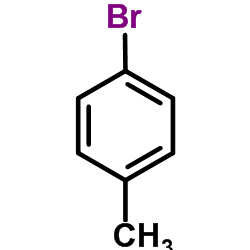
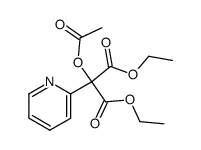
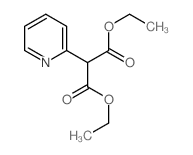
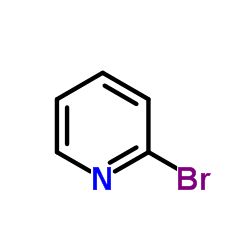
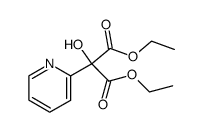
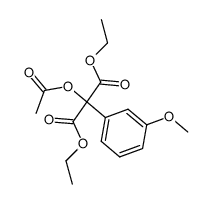
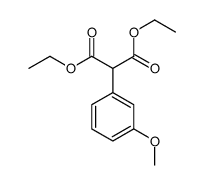
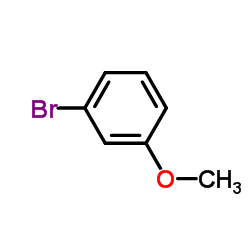
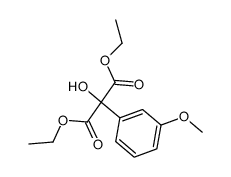
![diethyl 2-[4-(2-methylpropyl)phenyl]propanedioate Structure](https://image.chemsrc.com/caspic/249/23197-72-0.png)