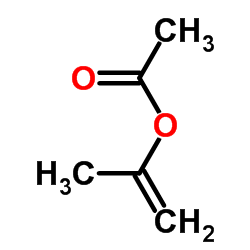
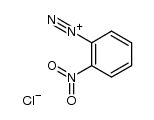
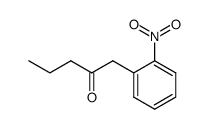
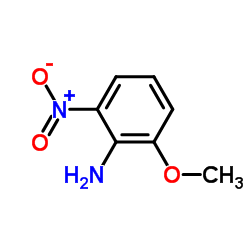
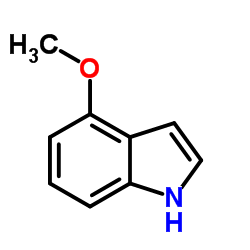
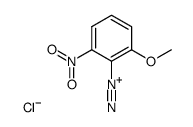
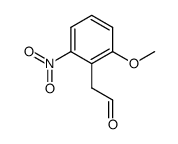
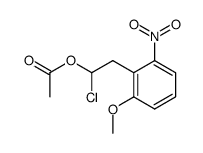
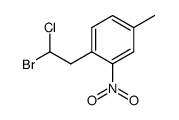
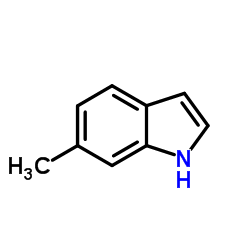
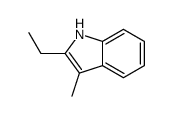
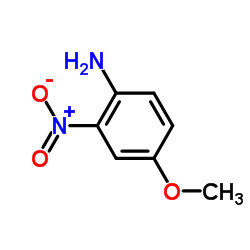
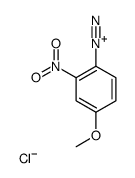
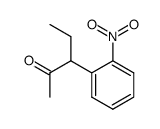
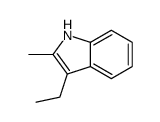
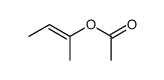
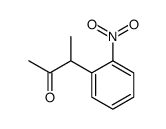
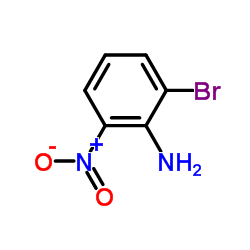
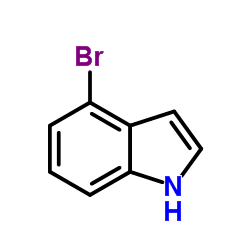
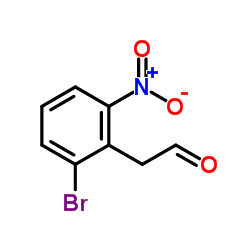
|
~76% |
|
~% |
|
~64% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~70% |
|
~54% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~53% |
|
~% |
|
~% |
|
~13% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |