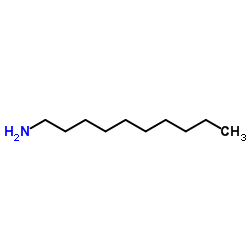
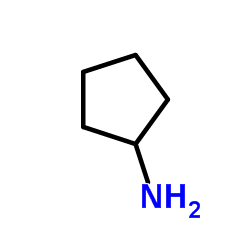
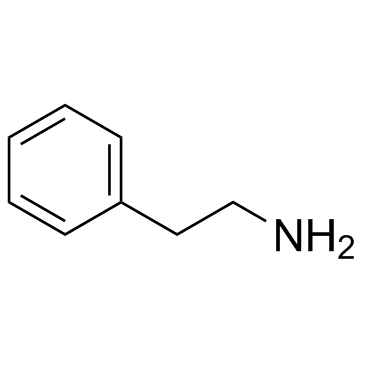
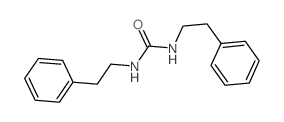
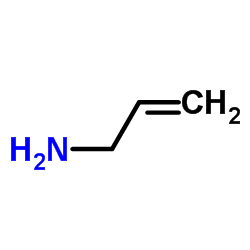
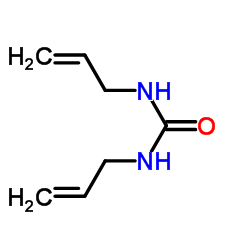
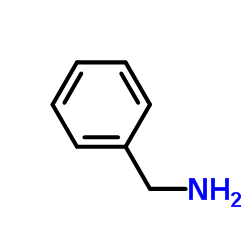
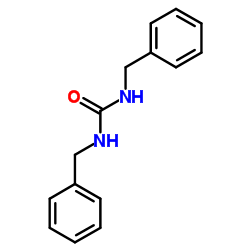
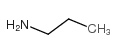
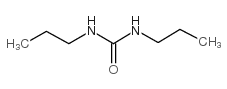
|
~68% |
|
~42% |
|
~50% |
|
~27% |
|
~26% |
|
~45% |