|
~% |
|
~% |
|
~% |
|
~% |
![2-[chloro(propan-2-yl)phosphoryl]propane Structure](https://image.chemsrc.com/caspic/275/1112-15-8.png)
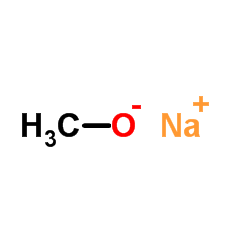
![2-[methoxy(propan-2-yl)phosphoryl]propane Structure](https://image.chemsrc.com/caspic/047/18632-46-7.png)
![1-[butyl(chloro)phosphoryl]butane Structure](https://image.chemsrc.com/caspic/413/683-16-9.png)
![1-[butyl(methoxy)phosphoryl]butane Structure](https://image.chemsrc.com/caspic/023/7163-67-9.png)


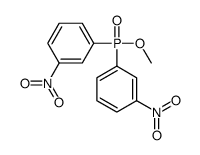
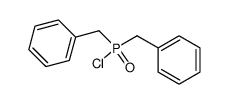
![[benzyl(methoxy)phosphoryl]methylbenzene Structure](https://image.chemsrc.com/caspic/214/21713-63-3.png)