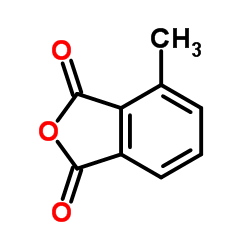
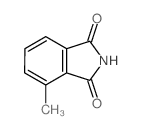
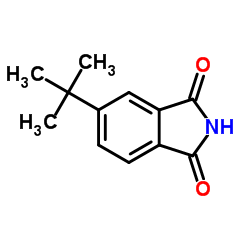
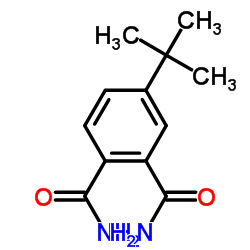
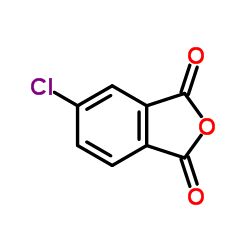
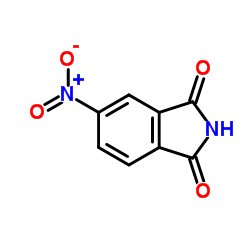
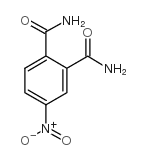
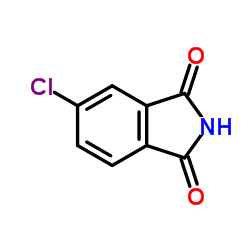
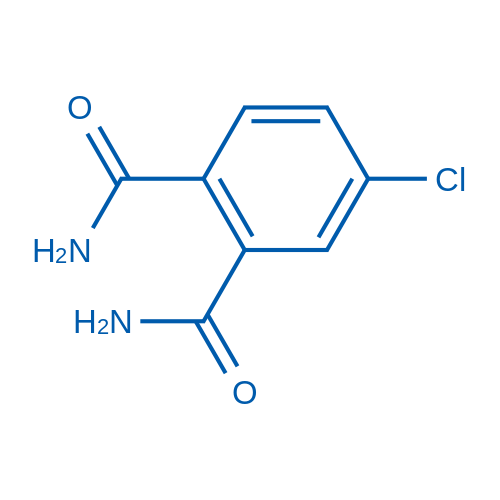
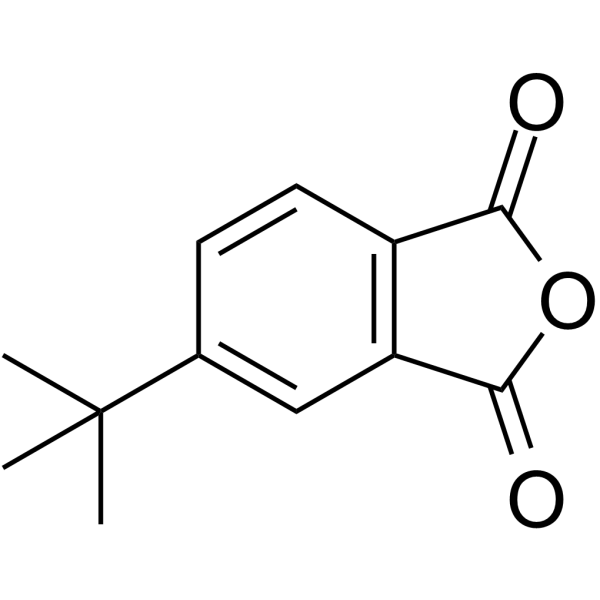
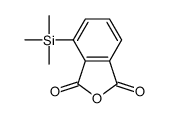
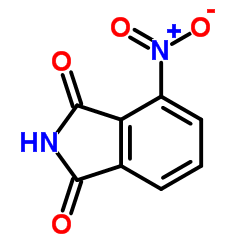
|
~70% |
|
~% |
|
~79% |
|
~72% |
|
~99% |
|
~71% |
|
~93% |
|
~% |
|
~% |
|
~93% |