|
~92% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~73% |
|
~% |
|
~85% |
|
~83% |
|
~% |
![(5,6-dihydrobenzo[k]tetraphen-7-yl)(methyl)sulfane Structure](https://image.chemsrc.com/caspic/165/134576-18-4.png)


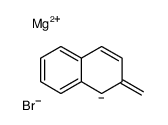
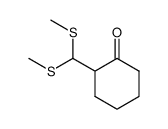

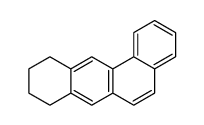
![5,6-Dihydrobenz[a]anthracene Structure](https://image.chemsrc.com/caspic/152/36914-99-5.png)
![Benzo[k]tetraphene Structure](https://image.chemsrc.com/caspic/062/53-70-3.png)
![7-(Methylthio)-5,6-dihydrodibenz[a,j]anthracene Structure](https://image.chemsrc.com/caspic/279/134576-15-1.png)
![Benzo[m]tetraphene Structure](https://image.chemsrc.com/caspic/094/224-41-9.png)

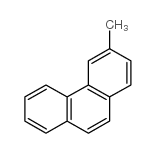
![5,6-Dihydrodibenz[a,j]anthracene Structure](https://image.chemsrc.com/caspic/390/16361-01-6.png)