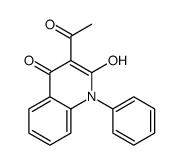
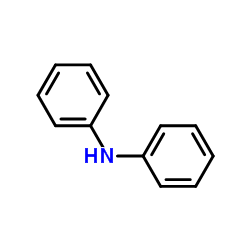
|
~93% |
|
~91% |
|
~% |
|
~% |
|
~% |
|
~92% |
|
~96% |
|
~% |
|
~% |
|
~93% |
|
~% |
|
~% |
|
~% |
|
~91% |
|
~% |
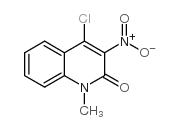
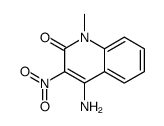
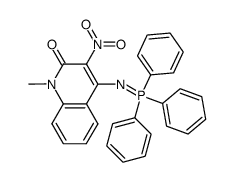

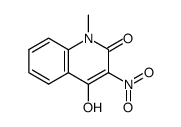
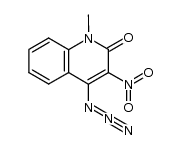


![5-methyl-3-oxido-[1,2,5]oxadiazolo[3,4-c]quinolin-3-ium-4-one Structure](https://image.chemsrc.com/caspic/214/141945-42-8.png)
![4-hydroxy-6-phenyl-2H-pyrano[3,2-c]quinoline-2,5(6H)-dione Structure](https://image.chemsrc.com/caspic/100/18706-64-4.png)