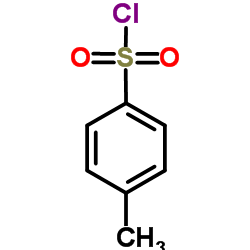
|
~75% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

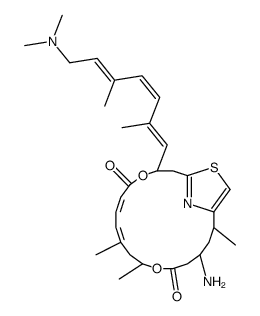
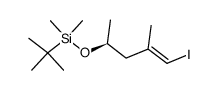

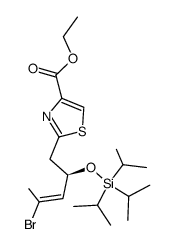

![tert-butyl ((3S,11S,15R,17S,E)-3-((E)-2-bromoprop-1-en-1-yl)-9,11,17-trimethyl-5,13-dioxo-4,12-dioxa-20-thia-21-azabicyclo[16.2.1]henicosa-1(21),8,18-trien-6-yn-15-yl)carbamate Structure](https://image.chemsrc.com/caspic/131/201340-07-0.png)