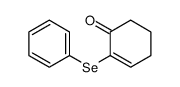
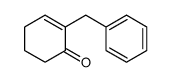
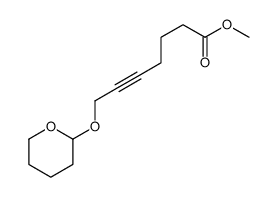
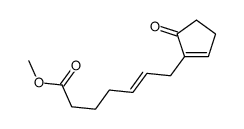
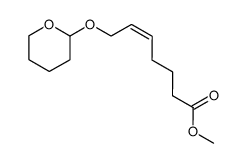
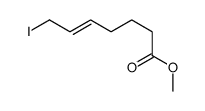
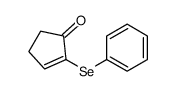
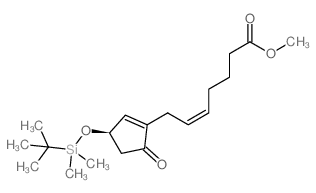
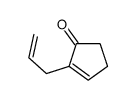
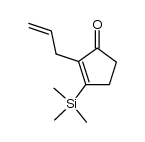
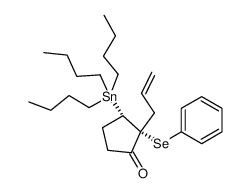
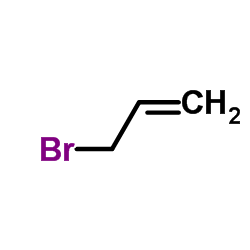
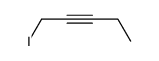
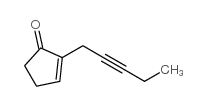
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~45% |
|
~99% |
|
~% |
|
~% |
|
~% |
|
~% |