|
~90% |
|
~94% |
|
~% |
|
~% |
|
~% |

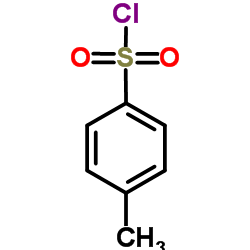


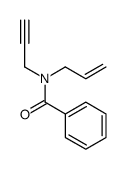
![2-tosylhexahydrocyclopenta[c]pyrrol-5(1H)-one Structure](https://image.chemsrc.com/caspic/052/133886-44-9.png)
![2-TOSYL-2,3,3A,4-TETRAHYDROCYCLOPENTA[C]PYRROL-5(1H)-ONE Structure](https://image.chemsrc.com/caspic/090/133886-43-8.png)