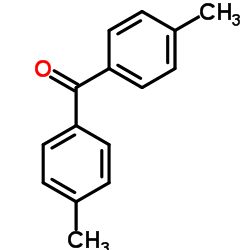
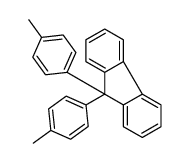
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
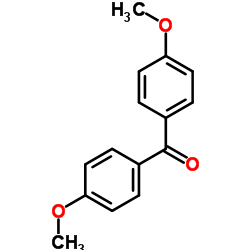


![[1,1'-biphenyl]-2-ylbis(4-methoxyphenyl)methanol Structure](https://image.chemsrc.com/caspic/011/16513-88-5.png)