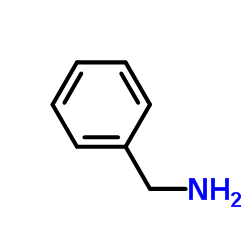
|
~% |
|
~% |
|
~37% |
|
~47% |
|
~% |
|
~% |

![[(2S,5R)-5-[6-(methylamino)purin-9-yl]-2,5-dihydrofuran-2-yl]methanol Structure](https://image.chemsrc.com/caspic/395/117723-57-6.png)
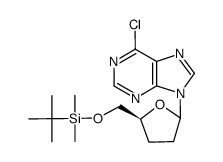
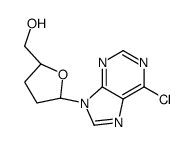
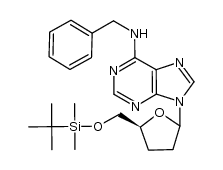
![[(2S,5R)-5-[6-(benzylamino)purin-9-yl]oxolan-2-yl]methanol Structure](https://image.chemsrc.com/caspic/449/120503-63-1.png)