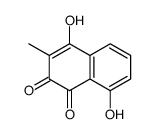
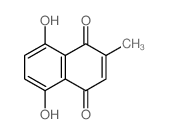
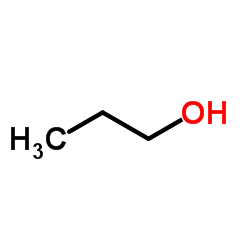
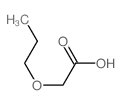
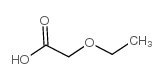
|
~58% |
|
~% |
|
~24% |
|
~26% |
|
~25% |
|
~45% |
|
~% |
|
~% |
|
~25% |
|
~% |
|
~62% |
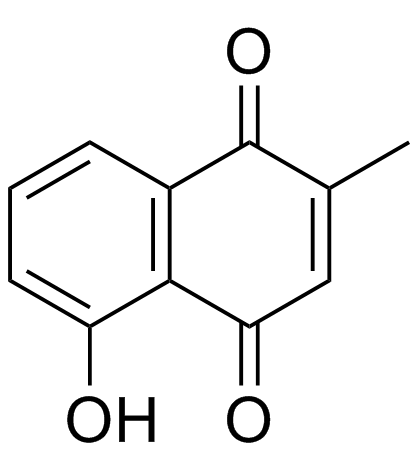
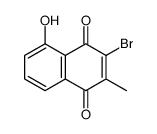
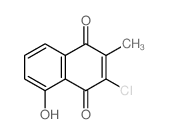
![3-hydroxy-7a-methyl-1aH-naphtho[2,3-b]oxirene-2,7-dione Structure](https://image.chemsrc.com/caspic/151/188675-38-9.png)
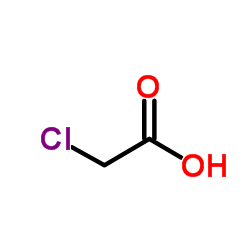
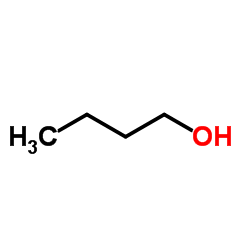
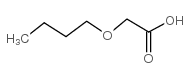
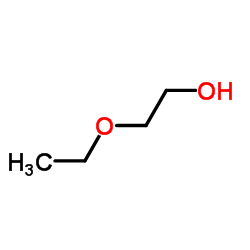
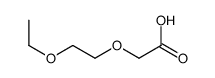
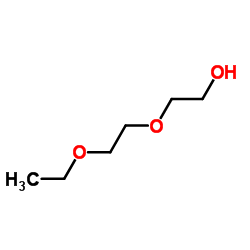
![2-[2-(2-ethoxyethoxy)ethoxy]acetic acid Structure](https://image.chemsrc.com/caspic/412/7743-98-8.png)