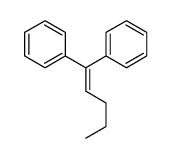
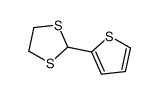
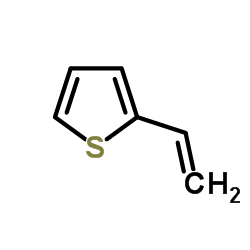
|
~94% |
|
~% |
|
~83% |
|
~58% |
|
~% |
|
~93% |
|
~60% |
|
~71% |
|
~84% |
|
~60% |
|
~41% |
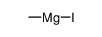
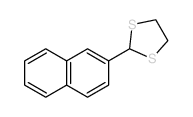
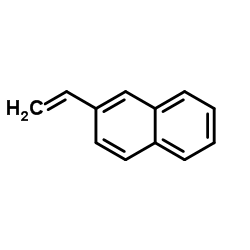
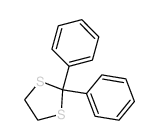

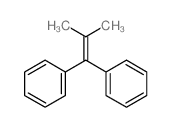
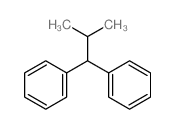

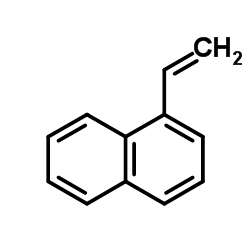
![2-[4-(1,3-dithiolan-2-yl)phenyl]-1,3-dithiolane Structure](https://image.chemsrc.com/caspic/054/69922-37-8.png)
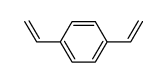

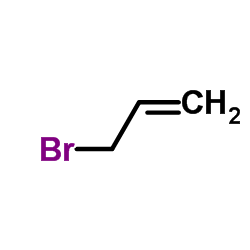


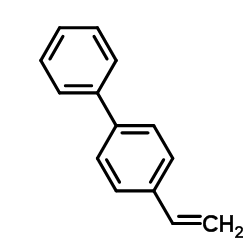

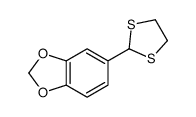
![5-VINYLBENZO[D][1,3]DIOXOLE Structure](https://image.chemsrc.com/caspic/407/7315-32-4.png)