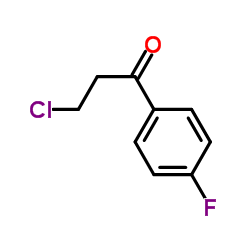
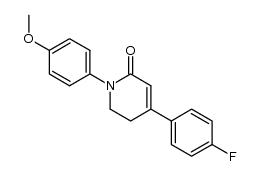
|
~% |
|
~51% |
|
~81% |
|
~% |
|
~82% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
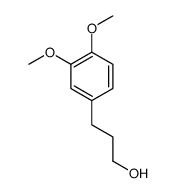
![(4R)-4-[(3,4-dimethoxyphenyl)methyl]oxolan-2-one Structure](https://image.chemsrc.com/caspic/386/108102-77-8.png)
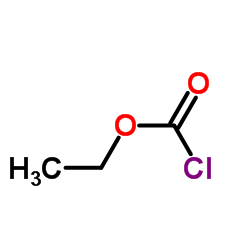
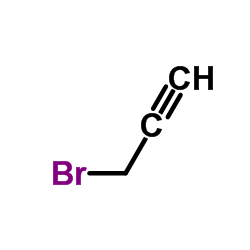

![ethyl 4-[bis(trimethylsilyl)amino]but-2-ynoate Structure](https://image.chemsrc.com/caspic/105/91387-29-0.png)


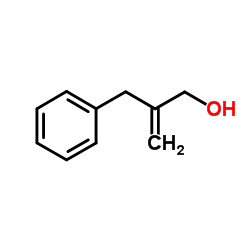
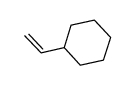
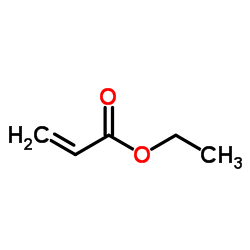
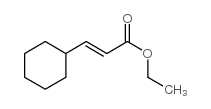
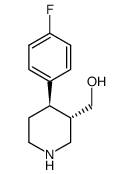
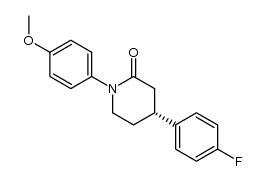
![[(3S,4R)-4-(4-fluorophenyl)-1-(4-methoxyphenyl)-piperidin-3-yl]methanol Structure](https://image.chemsrc.com/caspic/359/607375-35-9.png)