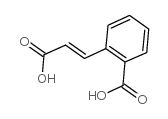
|
~% |
|
~24% |
|
~% |
|
~66% |
|
~96% |
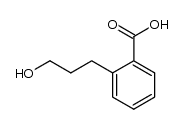

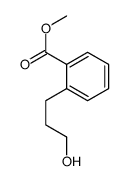

silane Structure](https://image.chemsrc.com/caspic/267/37891-79-5.png)