|
~73% |
|
~38% |
|
~75% |
|
~% |
|
~% |
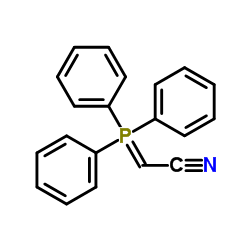
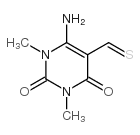
![7-amino-1,3-dimethylpyrido[2,3-d]pyrimidine-2,4-dione Structure](https://image.chemsrc.com/caspic/425/52294-86-7.png)
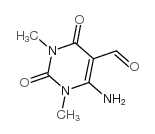

![8,10-dimethyl-2,8,10-triazabicyclo[4.4.0]deca-4,11-diene-3,7,9-trione Structure](https://image.chemsrc.com/caspic/179/57821-20-2.png)