|
~51% |
|
~59% |
|
~43% |
|
~20% |

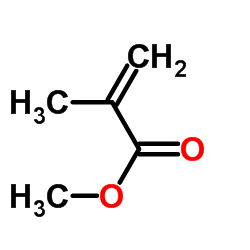

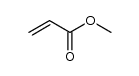

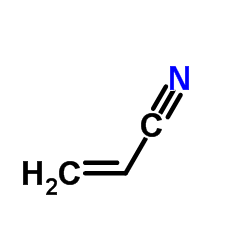


![5-[(2-Methyl-2-propanyl)oxy]-5-oxopentanoic acid Structure](https://image.chemsrc.com/caspic/374/63128-51-8.png)