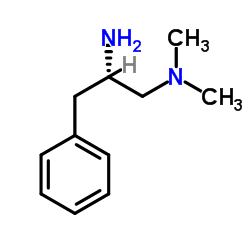
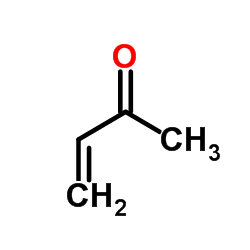
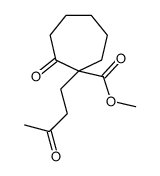
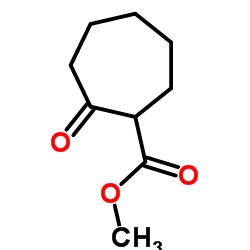
|
~85% |
|
~% |
|
~73% |
|
~65% |
|
~% |
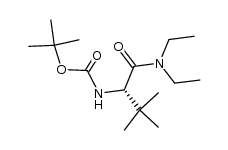
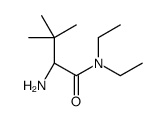
![tert-butyl (S)-N-[1-benzyl-2-(dimethylamino)ethyl]carbamate Structure](https://image.chemsrc.com/caspic/027/340161-69-5.png)