Evaluation of an external initiating Ni(II) diimine catalyst for electron-deficient π-conjugated polymers
Adam A. Pollit, Nimrat K. Obhi, Alan J. Lough, Dwight S. Seferos
Index: 10.1039/C7PY00873B
Full Text: HTML
Abstract
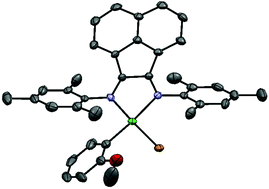
We have prepared, isolated, and evaluated the first Ni(II) diimine catalyst able to externally initiate the Kumada catalyst transfer polymerization of an electron-deficient π-conjugated monomer. The catalyst installs an o-methoxyphenyl group at the initiating end of polymer chains, providing a handle for characterization by 1H NMR and MALDI-ToF. External initiation provides a unique means to investigate the limits of polymerization control using Ni(II) diimines, which has not been possible using existing catalysts. The polymerization is chain-growth, however catalyst dissociation and chain reinitiation are increasingly competitive with polymer propagation at high molecular weight. This work provides the first mechanistic understanding of Kumada catalyst transfer polymeriation using Ni(II) diimine catalysts, and highlights the significant contributions of both catalyst and monomer species towards obtaining polymerization control.
|
Cholesterol Functionalized Aliphatic N-Substituted 8-Membere...
2018-04-10 [10.1039/C8PY00406D] |
|
Hollow polymer nanocapsules: synthesis, properties, and appl...
2018-04-10 [10.1039/C8PY00142A] |
|
Synthesis, Properties, and Crystallization of Alternating St...
2018-04-10 [10.1039/C8PY00391B] |
|
Tailored microstructure and mechanical properties of nanocom...
2018-04-09 [10.1039/C8PY00268A] |
|
Combining uretdione and disulfide reversibly degradable poly...
2018-04-09 [10.1039/C7PY01978E] |