|
~% |
|
~% |
|
~% |
|
~43% |
|
~% |
|
~% |
|
~% |
|
~88% |
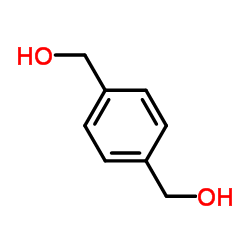
![(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis[4-(hydroxymethyl)benzyl]-1,3-diazepan-2-one Structure](https://image.chemsrc.com/caspic/234/151867-81-1.png)

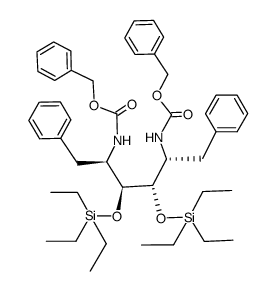
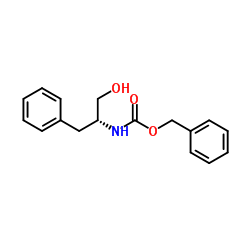
![Benzyl [(2R)-1-oxo-3-phenyl-2-propanyl]carbamate Structure](https://image.chemsrc.com/caspic/297/63219-70-5.png)