|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~% |
|
~% |
|
~53% |
|
~% |
|
~% |
|
~% |
|
~80% |
![4-ethyl-4,6,7,8-tetrahydro-1H-pyrano[3,4-f]indolizine-3,10-dione Structure](https://image.chemsrc.com/caspic/460/43083-10-9.png)
![(±)- 1H-Pyrano[3,4-f]indolizine-3,6,10(4H)-trione, 4-ethyl-7,8-dihydro-4-hydroxy Structure](https://image.chemsrc.com/caspic/342/102978-40-5.png)
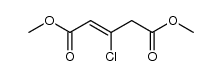
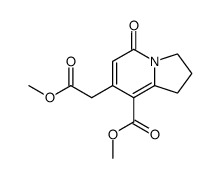
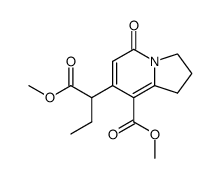
![methyl 4-ethyl-3,10-dioxo-3,4,6,7,8,10-hexahydro-1H-pyrano[3,4-f]indolizine-5-carboxylate Structure](https://image.chemsrc.com/caspic/065/145474-08-4.png)
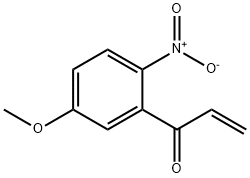


![1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione,4-ethyl-4-hydroxy Structure](https://image.chemsrc.com/caspic/436/31456-25-4.png)