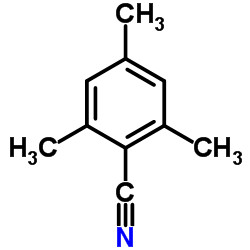
|
~64% |
|
~% |
|
~34% |
|
~% |
|
~% |
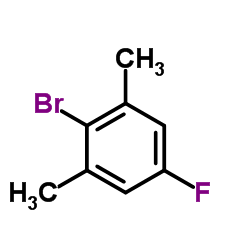
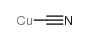
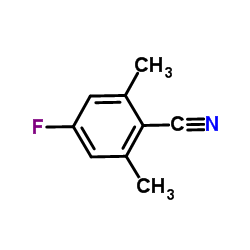
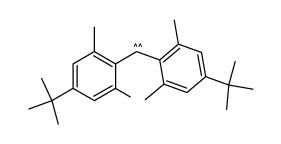
![5-tert-butyl-2-[(4-tert-butyl-2,6-dimethylphenyl)methyl]-1,3-dimethylbenzene Structure](https://image.chemsrc.com/caspic/381/65338-71-8.png)
![2-[diazo-(2,4,6-trimethylphenyl)methyl]-1,3,5-trimethylbenzene Structure](https://image.chemsrc.com/caspic/264/61080-14-6.png)