|
~68% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~95% |
|
~% |
|
~% |
|
~% |
|
~74% |
|
~% |
|
~95% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
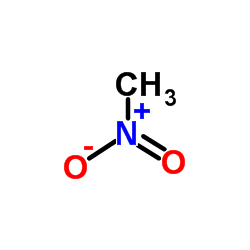
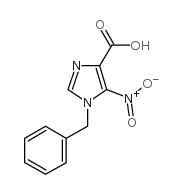

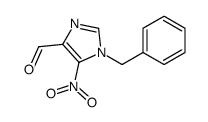
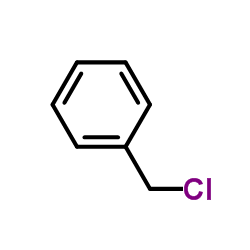

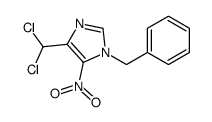
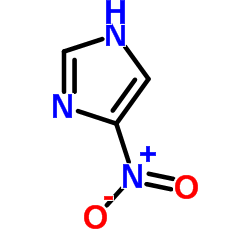

![2-amino-1-[5-amino-1-(phenylmethyl)-1h-imidazol-4-yl] ethanone Structure](https://image.chemsrc.com/caspic/363/69195-91-1.png)