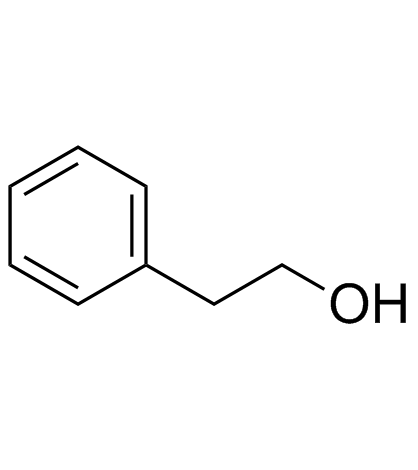
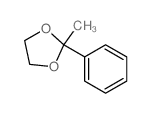
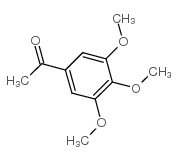
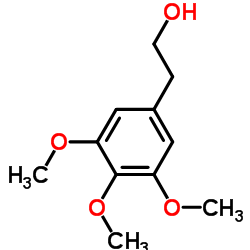
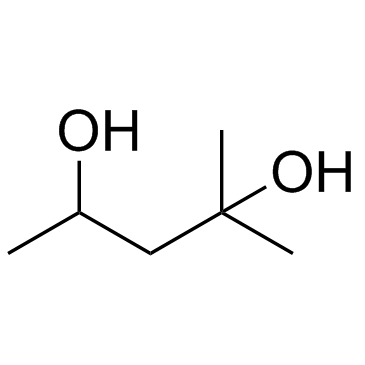
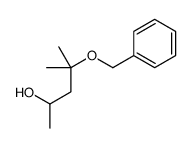
|
~72% |
|
~% |
|
~% |
|
~51% |
|
~61% |
|
~82% |
|
~79% |
|
~24% |
|
~% |
|
~% |
|
~% |
|
~% |
![9-(2-methylpropyl)-9-borabicyclo[3.3.1]nonane Structure](https://image.chemsrc.com/caspic/470/63942-77-8.png)
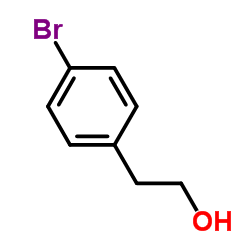
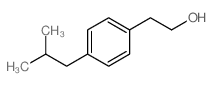
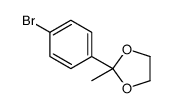
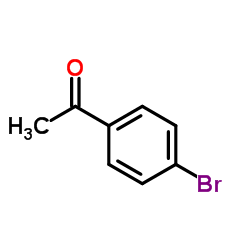
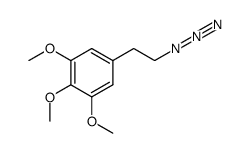
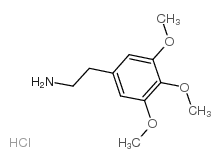
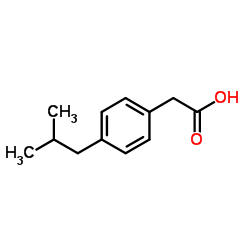
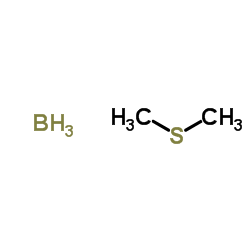
![[1E,5Z,pR]-1,5-Cyclooctadiene Structure](https://image.chemsrc.com/caspic/193/5259-72-3.png)
![9,9'-Bi(9-borabicyclo[3.3.1]nonane) Structure](https://image.chemsrc.com/caspic/204/21205-91-4.png)