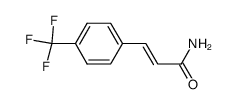
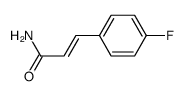
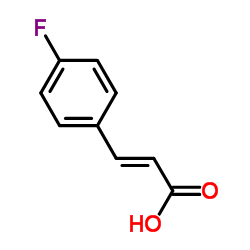
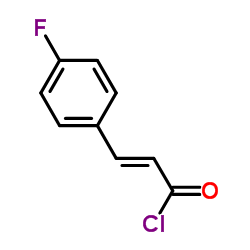
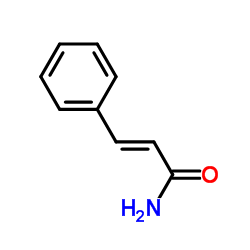
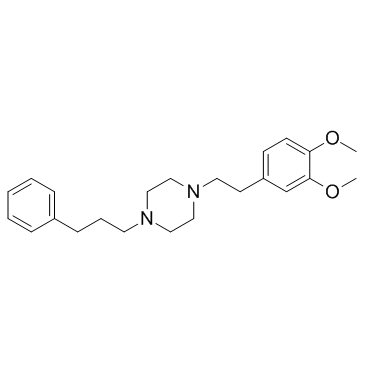
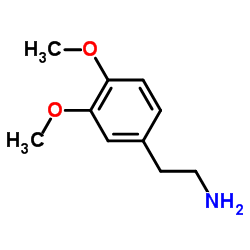
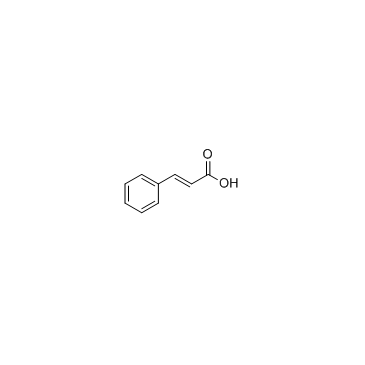
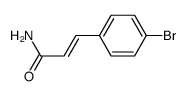
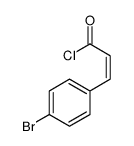
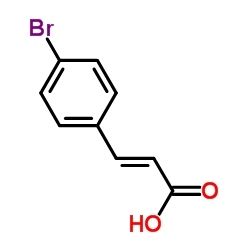
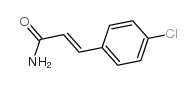
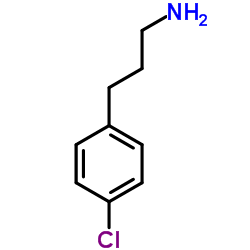
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
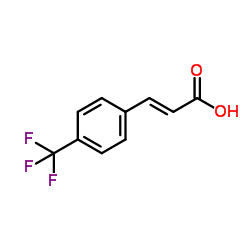
![3-[4-(Trifluoromethyl)phenyl]-1-propanamine Structure](https://image.chemsrc.com/caspic/186/101488-60-2.png)
![3-[4-(trifluoromethyl)phenyl]prop-2-enoyl chloride Structure](https://image.chemsrc.com/caspic/439/120681-07-4.png)