|
~13% |
|
~58% |
|
~99% |
|
~98% |
|
~98% |
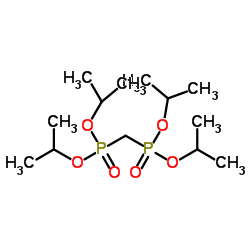

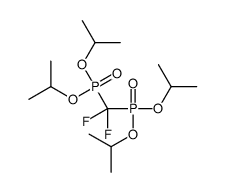
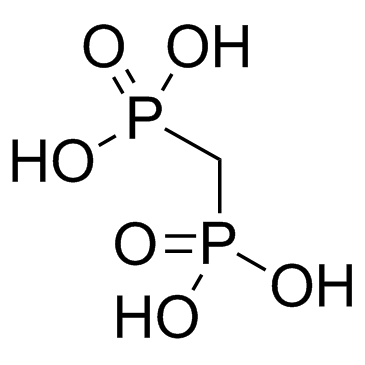
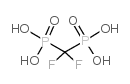
![[fluoro(phosphono)methyl]phosphonic acid Structure](https://image.chemsrc.com/caspic/340/10595-93-4.png)