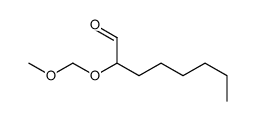
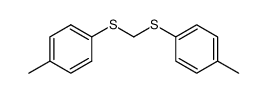
|
~10% |
|
~% |
|
~85% |
|
~% |
|
~% |
|
~% |
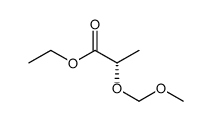
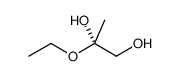


![1-[2-(methoxymethoxy)-1-(4-methylphenyl)sulfanyloctyl]sulfanyl-4-methylbenzene Structure](https://image.chemsrc.com/caspic/295/91191-98-9.png)