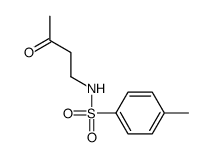
|
~86% |
|
~91% |
|
~% |
|
~% |
|
~86% |
|
~% |

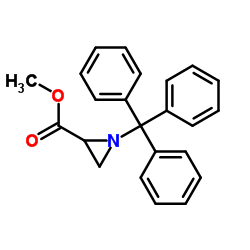
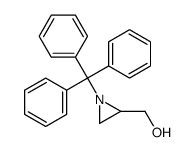
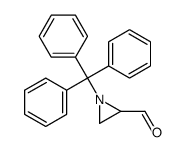
![[N-(p-Toluenesulfonyl)imino]phenyliodinane Structure](https://image.chemsrc.com/caspic/449/55962-05-5.png)