|
~71% |
|
~69% |
|
~58% |
|
~66% |
|
~76% |
|
~79% |
|
~70% |
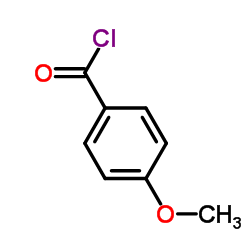

![[3-(4-methoxybenzoyl)phenyl]-(4-methoxyphenyl)methanone Structure](https://image.chemsrc.com/caspic/474/7477-29-4.png)


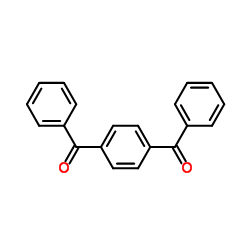

![[4-(4-methylbenzoyl)phenyl]-(4-methylphenyl)methanone Structure](https://image.chemsrc.com/caspic/280/61565-13-7.png)
![[4-(4-methoxybenzoyl)phenyl]-(4-methoxyphenyl)methanone Structure](https://image.chemsrc.com/caspic/026/15517-45-0.png)


