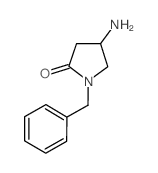
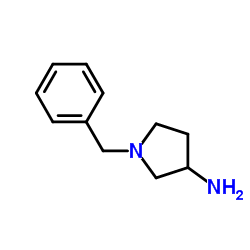
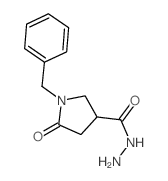
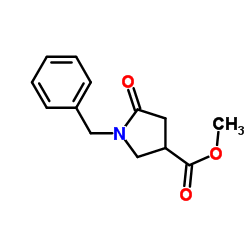
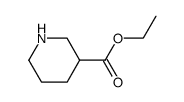
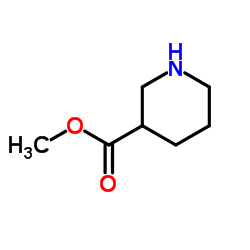
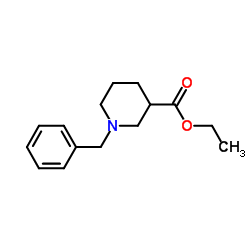
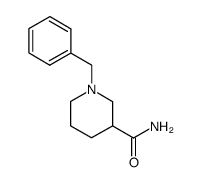
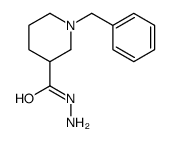
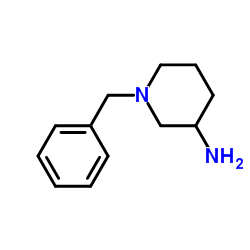
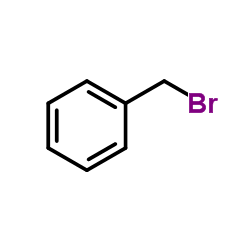
|
~80% |
|
~75% |
|
~72% |
|
~% |
|
~% |
|
~% |
|
~79% |
|
~93% |
|
~% |
|
~97% |
|
~% |
|
~71% |