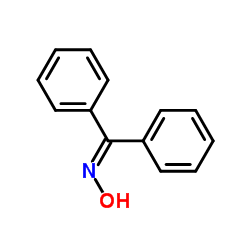
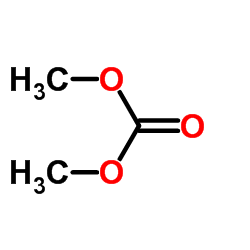
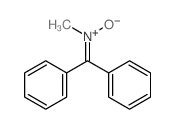
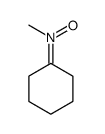
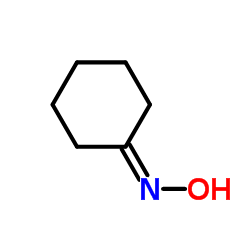
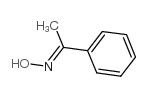
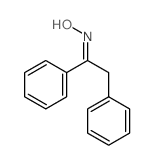
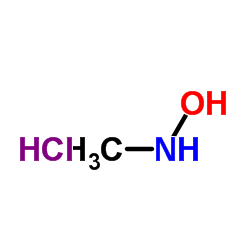
|
~24% |
|
~12%
Detail
|
|
~55% |
|
~44% |
|
~35% |
|
~31% |
|
~80% |