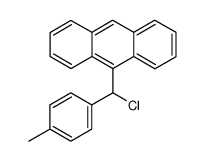
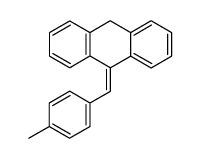
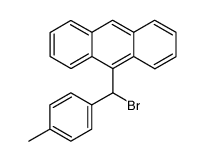
|
~95% |
|
~89% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~79% |
|
~95% |
|
~39% |
|
~% |
|
~% |
|
~% |


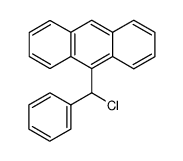
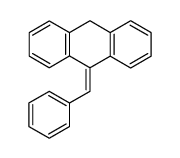
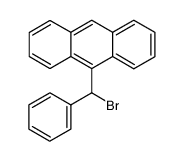
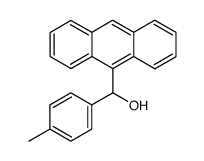
![9-[(4-methylphenyl)methyl]anthracene Structure](https://image.chemsrc.com/caspic/337/1498-79-9.png)