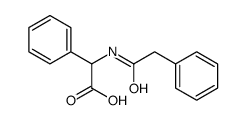
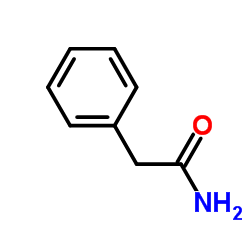
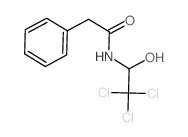
|
~73% |
|
~88% |
|
~91% |
|
~91% |
|
~88% |
|
~81% |
|
~95% |
|
~92% |
|
~71% |

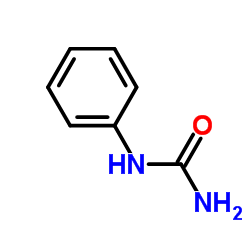
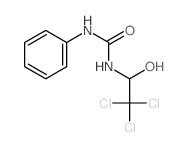
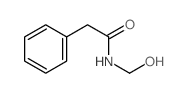

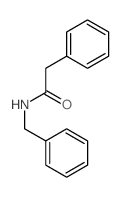
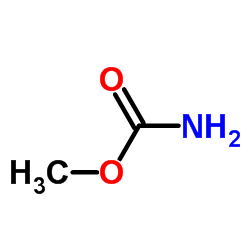

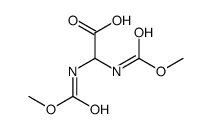
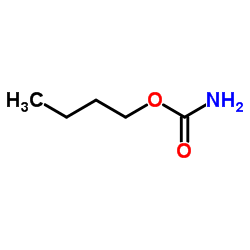
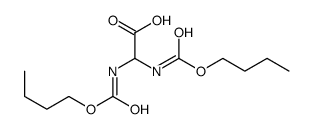

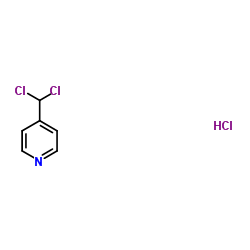
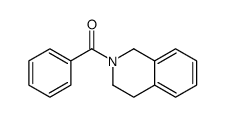

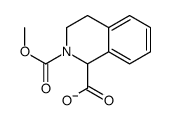
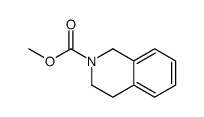
![2-hydroxy-2-[(2-phenylacetyl)amino]acetic acid Structure](https://image.chemsrc.com/caspic/133/56674-25-0.png)