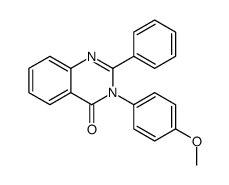
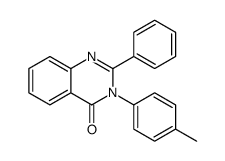
|
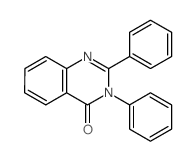
~73% |
|
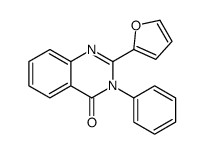
~84% |
|
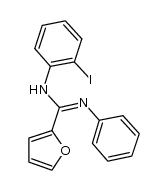
~12%
Detail
|
|
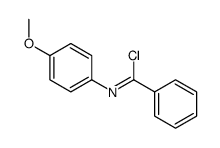
~79% |
|
~74% |