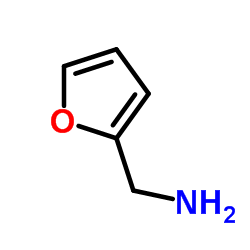
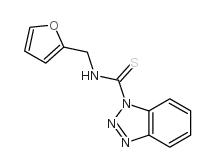
|
~90% |
|
~92% |
|
~99% |
|
~93% |
|
~97% |
|
~99% |
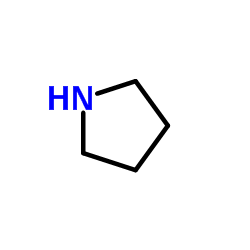

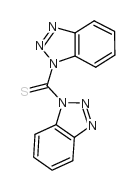
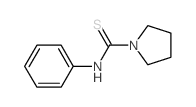
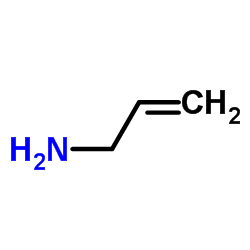
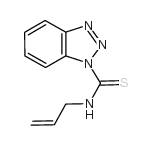
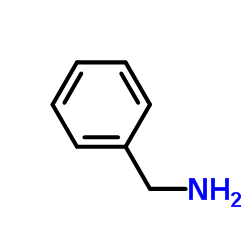
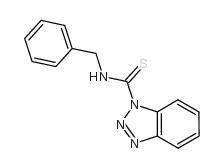

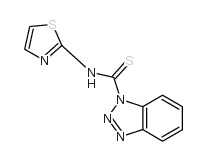

![1-[3-(dimethylamino)propyl]-3-phenylthiourea Structure](https://image.chemsrc.com/caspic/088/722-04-3.png)