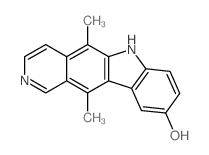
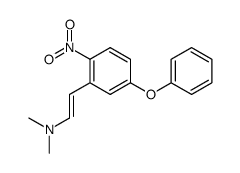
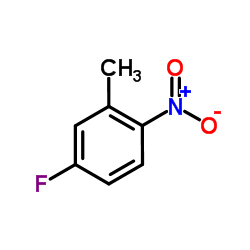
|
~67% |
|
~65% |
|
~% |
|
~% |
|
~% |
![6H-Pyrido[4,3-b]carbazole,9-methoxy-5,11-dimethyl Structure](https://image.chemsrc.com/caspic/093/10371-86-5.png)