几夫碱
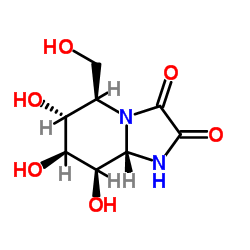
几夫碱结构式

|
常用名 | 几夫碱 | 英文名 | Kifunensine |
|---|---|---|---|---|
| CAS号 | 109944-15-2 | 分子量 | 232.191 | |
| 密度 | 1.9±0.1 g/cm3 | 沸点 | N/A | |
| 分子式 | C8H12N2O6 | 熔点 | >280℃ | |
| MSDS | 中文版 美版 | 闪点 | N/A |
几夫碱用途Kifunensine 是可从放线菌中分离得到的 I 类 α-mannosidases 有效选择性抑制剂,可阻断 α-mannosidases I 修剪糖蛋白上的甘露糖残基。Kifunensine 可抑制内质网相关蛋白降解途径。 |
| 中文名 | 几夫碱 |
|---|---|
| 英文名 | Kifunensine,(5R,6R,7S,8R,8aS)-Hexahydro-6,7,8-trihydroxy-5-(hydroxymethyl)-imidazo[1,2-a]pyridine-2,3-dione |
| 英文别名 | 更多 |
| 描述 | Kifunensine 是可从放线菌中分离得到的 I 类 α-mannosidases 有效选择性抑制剂,可阻断 α-mannosidases I 修剪糖蛋白上的甘露糖残基。Kifunensine 可抑制内质网相关蛋白降解途径。 |
|---|---|
| 相关类别 | |
| 体外研究 | Kifunensine是一种来自放线菌kifunense 9482的生物碱,是a-甘露糖苷酶I最有效的抑制剂,在an8GlcNAc2(Man8)或Man9GlcNAc2(Man9)阶段阻断N-聚糖的合成[3]。RT-PCR[3]。细胞系:表达人IgG1单克隆抗体的杂交瘤细胞[3]。浓度:2μg/mL,培养时间:4天。结果:扁豆凝集素结合明显降低。在所测试的抑制剂中,Kifunensine是最有效的产生抗体的低聚甘露糖残基不含岩藻糖。 |
| 体内研究 | 动物模型:BALB/c小鼠[3]。剂量:5mg/kg。给药:尾静脉注射。结果:用或不加kifunensine处理细胞产生的抗temb单克隆抗体在7d内无明显差异。 |
| 参考文献 |
| 密度 | 1.9±0.1 g/cm3 |
|---|---|
| 熔点 | >280℃ |
| 分子式 | C8H12N2O6 |
| 分子量 | 232.191 |
| 精确质量 | 232.069534 |
| PSA | 130.33000 |
| LogP | -2.21 |
| InChIKey | OIURYJWYVIAOCW-PQMKYFCFSA-N |
| SMILES | O=C1NC2C(O)C(O)C(O)C(CO)N2C1=O |
| 折射率 | 1.706 |
| 储存条件 | -20°C |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 危害码 (欧洲) | Xi |
| 危险品运输编码 | NONH for all modes of transport |
| 几夫碱上游产品 10 | |
|---|---|
| 几夫碱下游产品 1 | |
|
A context-independent N-glycan signal targets the misfolded extracellular domain of Arabidopsis STRUBBELIG to endoplasmic-reticulum-associated degradation.
Biochem. J. 464(3) , 401-11, (2014) N-glycosylation of proteins plays an important role in the determination of the fate of newly synthesized glycoproteins in the endoplasmic reticulum (ER). Specific oligosaccharide structures recruit m... |
|
|
Synthesis of kifunensine thioanalogs and their inhibitory activities against HIV-RT and α-mannosidase.
Carbohydr. Res. 365 , 1-8, (2013) An efficient and practical synthesis of kifunensine thioanalogs 1a-c was reported. The bicyclic azasugars fused thiazolidin-4-one 4a-c as key intermediates were first synthesized in good yields of 74-... |
|
|
Evolution of cross-neutralizing antibody specificities to the CD4-BS and the carbohydrate cloak of the HIV Env in an HIV-1-infected subject.
PLoS ONE 7(11) , e49610, (2012) Broadly neutralizing antibodies are considered an important part of a successful HIV vaccine. A better understanding of the factors underlying their development during infection and of the epitopes th... |
| (5R,6R,7S,8R,8aS)-6,7,8-Trihydroxy-5-(hydroxymethyl)hexahydroimidazo[1,2-a]pyridine-2,3-dione |
| Imidazo[1,2-a]pyridine-2,3-dione, hexahydro-6,7,8-trihydroxy-5-(hydroxymethyl)-, (5R,6R,7S,8R,8aS)- |
| KIFUNENSINE,KITASATOSPORIA KIFUNENSE |
| 1-deoxymannojirimycin |
| Kifunensine |

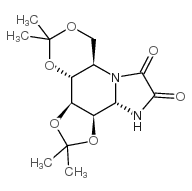 CAS号134234-43-8
CAS号134234-43-8 CAS号128741-75-3
CAS号128741-75-3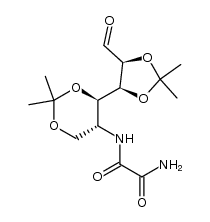 CAS号128741-70-8
CAS号128741-70-8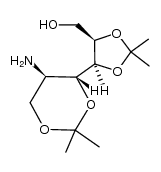 CAS号149819-14-7
CAS号149819-14-7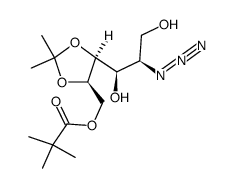 CAS号872038-08-9
CAS号872038-08-9 CAS号872037-91-7
CAS号872037-91-7 CAS号872037-89-3
CAS号872037-89-3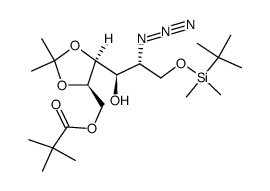 CAS号872037-90-6
CAS号872037-90-6 CAS号118464-47-4
CAS号118464-47-4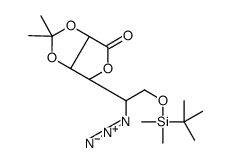 CAS号118464-49-6
CAS号118464-49-6
