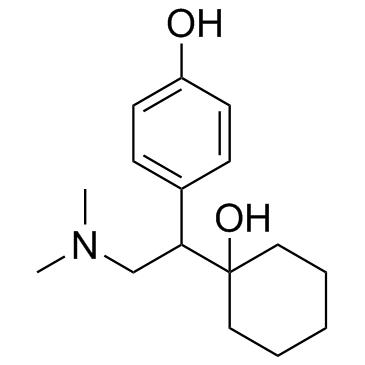
Venlafaxine hydrochloride
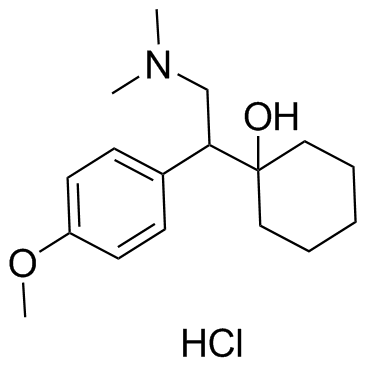
Venlafaxine hydrochloride structure
|
Common Name | Venlafaxine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 99300-78-4 | Molecular Weight | 313.863 | |
| Density | 1.394 g/cm3 | Boiling Point | 397.6ºC at 760 mmHg | |
| Molecular Formula | C17H28ClNO2 | Melting Point | 207-209ºC | |
| MSDS | USA | Flash Point | 194.2ºC | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of Venlafaxine hydrochlorideVenlafaxine hydrochloride is an antidepressant of the serotonin-norepinephrine reuptake inhibitor (SNRI) class.Target: SNRIVenlafaxine is an antidepressant of the serotonin-norepinephrine reuptake inhibitor (SNRI) class. First introduced by Wyeth in 1993, now marketed by Pfizer, it is licensed for the treatment of major depressive disorder (MDD), as a treatment for generalized anxiety disorder, and comorbid indications in certain anxiety disorders with depression. In 2007, venlafaxine was the sixth most commonly prescribed antidepressant on the U.S. retail market, with 17.2 million prescriptions.Venlafaxine is a bicyclic antidepressant, and usually categorized as a serotonin-norepinephrine reuptake inhibitor (SNRI), but it has been referred to as a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI). It works by blocking the transporter "reuptake" proteins for key neurotransmitters affecting mood, thereby leaving more active neurotransmitters in the synapse. The neurotransmitters affected are serotonin and norepinephrine. Additionally, in high doses it weakly inhibits the reuptake of dopamine, with recent evidence showing that the norepinephrine transporter also transports some dopamine as well, since dopamine is inactivated by norepinephrine reuptake in the frontal cortex. The frontal cortex largely lacks dopamine transporters; therefore, venlafaxine can increase dopamine neurotransmission in this part of the brain. Venlafaxine interacts with opioid receptors (mu-, kappa1- kappa3- and delta-opioid receptor subtypes) as well as the alpha2-adrenergic receptor, and was shown to increase pain threshold in mice. When mice were tested with a hotplate analgesia meter (to measure pain), both venlafaxine and mirtazapine induced a dose-dependent, naloxone-reversible antinociceptive effect following intraperitoneal injection. These findings suggest venlafaxine's seemingly superior efficacy in severe depression as narcotics become increasingly used as a measure of last resort for refractory cases. |
| Name | venlafaxine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Venlafaxine hydrochloride is an antidepressant of the serotonin-norepinephrine reuptake inhibitor (SNRI) class.Target: SNRIVenlafaxine is an antidepressant of the serotonin-norepinephrine reuptake inhibitor (SNRI) class. First introduced by Wyeth in 1993, now marketed by Pfizer, it is licensed for the treatment of major depressive disorder (MDD), as a treatment for generalized anxiety disorder, and comorbid indications in certain anxiety disorders with depression. In 2007, venlafaxine was the sixth most commonly prescribed antidepressant on the U.S. retail market, with 17.2 million prescriptions.Venlafaxine is a bicyclic antidepressant, and usually categorized as a serotonin-norepinephrine reuptake inhibitor (SNRI), but it has been referred to as a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI). It works by blocking the transporter "reuptake" proteins for key neurotransmitters affecting mood, thereby leaving more active neurotransmitters in the synapse. The neurotransmitters affected are serotonin and norepinephrine. Additionally, in high doses it weakly inhibits the reuptake of dopamine, with recent evidence showing that the norepinephrine transporter also transports some dopamine as well, since dopamine is inactivated by norepinephrine reuptake in the frontal cortex. The frontal cortex largely lacks dopamine transporters; therefore, venlafaxine can increase dopamine neurotransmission in this part of the brain. Venlafaxine interacts with opioid receptors (mu-, kappa1- kappa3- and delta-opioid receptor subtypes) as well as the alpha2-adrenergic receptor, and was shown to increase pain threshold in mice. When mice were tested with a hotplate analgesia meter (to measure pain), both venlafaxine and mirtazapine induced a dose-dependent, naloxone-reversible antinociceptive effect following intraperitoneal injection. These findings suggest venlafaxine's seemingly superior efficacy in severe depression as narcotics become increasingly used as a measure of last resort for refractory cases. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.394 g/cm3 |
|---|---|
| Boiling Point | 397.6ºC at 760 mmHg |
| Melting Point | 207-209ºC |
| Molecular Formula | C17H28ClNO2 |
| Molecular Weight | 313.863 |
| Flash Point | 194.2ºC |
| Exact Mass | 313.180847 |
| PSA | 32.70000 |
| LogP | 3.83760 |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-37/39 |
| RIDADR | 1230.0 |
| RTECS | GV8872760 |
| Hazard Class | 3、6.1 |
| HS Code | 2922509090 |
|
~96% 
Venlafaxine hyd... CAS#:99300-78-4 |
| Literature: AARTI HEALTHCARE LIMITED Patent: WO2007/69277 A2, 2007 ; Location in patent: Page/Page column 12-15 ; |
|
~95% 
Venlafaxine hyd... CAS#:99300-78-4 |
| Literature: Siegfried Ltd. Patent: EP2072495 A1, 2009 ; Location in patent: Page/Page column 9 ; |
|
~% 
Venlafaxine hyd... CAS#:99300-78-4 |
| Literature: US2010/94055 A1, ; Page/Page column 4-5 ; |
|
~% 
Venlafaxine hyd... CAS#:99300-78-4 |
| Literature: EP1721889 A1, ; Page/Page column 6 ; |
|
~78% 
Venlafaxine hyd... CAS#:99300-78-4 |
| Literature: TEVA PHARMACEUTICAL INDUSTRIES LTD.; TEVA PHARMACEUTICALS USA, INC. Patent: WO2007/47972 A2, 2007 ; Location in patent: Page/Page column 16; 17 ; |
|
~% 
Venlafaxine hyd... CAS#:99300-78-4 |
| Literature: WO2007/49302 A2, ; Page/Page column 7-8 ; |
|
~% 
Venlafaxine hyd... CAS#:99300-78-4 |
| Literature: Organic Process Research and Development, , vol. 15, # 6 p. 1392 - 1395 |
|
~% 
Venlafaxine hyd... CAS#:99300-78-4 |
| Literature: Organic Process Research and Development, , vol. 15, # 6 p. 1392 - 1395 |
|
~% 
Venlafaxine hyd... CAS#:99300-78-4 |
| Literature: Organic Process Research and Development, , vol. 15, # 6 p. 1392 - 1395 |
| Precursor 8 | |
|---|---|
| DownStream 3 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Environmental friendly method for urban wastewater monitoring of micropollutants defined in the Directive 2013/39/EU and Decision 2015/495/EU.
J. Chromatogr. A. 1418 , 140-9, (2015) The fate and removal of organic micropollutants in the environment is a demanding issue evidenced by the recent European policy. This work presents an analytical method for the trace quantification of... |
|
|
The antidepressant venlafaxine disrupts brain monoamine levels and neuroendocrine responses to stress in rainbow trout.
Environ. Sci. Technol. 48(22) , 13434-42, (2014) Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is a widely prescribed antidepressant drug routinely detected in the aquatic environment. However, little is known about its impact on the p... |
|
|
Enantiomeric fraction evaluation of pharmaceuticals in environmental matrices by liquid chromatography-tandem mass spectrometry.
J. Chromatogr. A. 1363 , 226-235, (2014) The interest for environmental fate assessment of chiral pharmaceuticals is increasing and enantioselective analytical methods are mandatory. This study presents an enantioselective analytical method ... |
| 1-[2-(Dimethylamino)-1-(4-methoxyphenyl)ethyl]cyclohexanol hydrochloride (1:1) |
| Efectin |
| Venlafaxine Hydrochloride |
| Effexor |
| Venlafaxine HCl |
| MFCD03658865 |
| EINECS 200-659-6 |
| Cyclohexanol, 1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]-, hydrochloride (1:1) |
| Efexor |
| (±)-1-(a-((Dimethylamino)methyl)-p-methoxybenzyl)cyclohexanol hydrochloride |
| cyclohexanol, 1-[2-(dimethylamino)-1-(4-methoxyphenyl)ethyl]-, hydrochloride |
| RTECS GV8872760 |
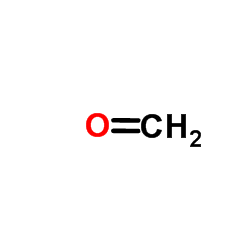
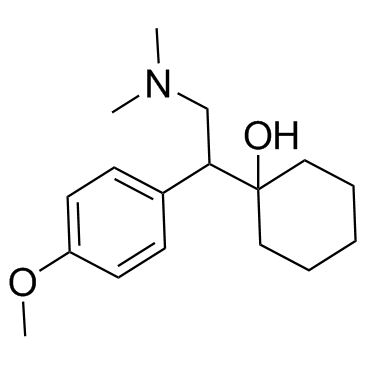
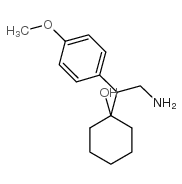
![1-[2-Amino-1-(4-methoxyphenyl)-ethyl]-cyclohexanol hydrochloride structure](https://www.chemsrc.com/caspic/172/130198-05-9.png)
![1-[Cyano-(p-methoxyphenyl)methyl]cyclohexanol structure](https://www.chemsrc.com/caspic/015/93413-76-4.png)
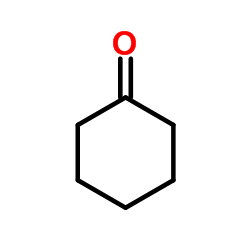
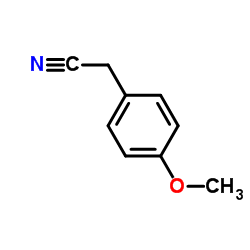
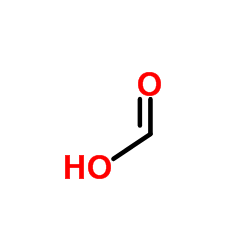
 CAS#:93413-62-8
CAS#:93413-62-8 CAS#:448904-47-0
CAS#:448904-47-0
