CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
UX9363950
-
CHEMICAL NAME :
-
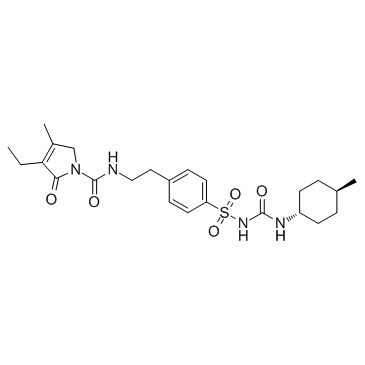
1H-Pyrrole-1-carboxamide, 2,5-dihydro-3-ethyl-4-methyl-N-(2-(4-(((((4-methylcyc lohexyl)amino) carbonyl)amino)sulfonyl)phenyl)ethyl)-2-oxo-, trans-
-
CAS REGISTRY NUMBER :
-
93479-97-1
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
5
-
MOLECULAR FORMULA :
-
C24-H34-N4-O5-S
-
MOLECULAR WEIGHT :
-
490.68
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Liver - other changes
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 43,547,1993
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>3950 mg/kg
-
TOXIC EFFECTS :
-
Liver - other changes
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 43,547,1993
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DIFREZ Diabetes Frontier. (Medikaru Rebyusha, Yoshida Bldg., 1-7-3 Hirano-machi, Chuo-ku, Osaka, 541, Japan) V.1- 1990- Volume(issue)/page/year: 3,565,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DIFREZ Diabetes Frontier. (Medikaru Rebyusha, Yoshida Bldg., 1-7-3 Hirano-machi, Chuo-ku, Osaka, 541, Japan) V.1- 1990- Volume(issue)/page/year: 3,565,1992 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
254 ug/kg
-
SEX/DURATION :
-
female 7-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - abortion Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 27,1477,1993
|