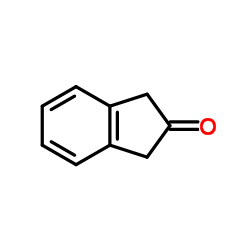
delapril hcl
Modify Date: 2024-01-04 20:56:12
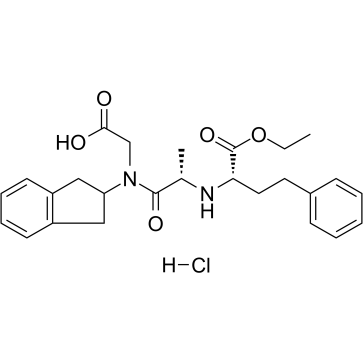
delapril hcl structure
|
Common Name | delapril hcl | ||
|---|---|---|---|---|
| CAS Number | 83435-67-0 | Molecular Weight | 489.004 | |
| Density | N/A | Boiling Point | 659.7ºC at 760 mmHg | |
| Molecular Formula | C26H33ClN2O5 | Melting Point | 169ºC | |
| MSDS | N/A | Flash Point | 352.7ºC | |
Use of delapril hclDelapril is an angiotensin-converting enzyme (ACE) inhibitor for the treatment of cardiovascular diseases[1]. |
| Name | Delapril Hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Delapril is an angiotensin-converting enzyme (ACE) inhibitor for the treatment of cardiovascular diseases[1]. |
|---|---|
| Related Catalog | |
| Target |
ACE[1] |
| In Vivo | Delapril (3 mg/kg; administered orally for 2 weeks) exerts potent ACE inhibitory activity in spontaneously hypertensive rat (SHR) [1]. Delapril (1-10 mg/kg; orally) exerts a marked and long-lasting antihypertensive action in various experimental models of hypertension[1]. Animal Model: SHR[1] Dosage: 3 mg/kg Administration: Administered orally; 2 weeks Result: Exerted potent ACE inhibitory activity. Animal Model: 2-kidney, 1-clip hypertensive Rats, Dogs, and SHR[1] Dosage: 1-10 mg/kg Administration: Oral Result: Exerted a marked and long-lasting antihypertensive action. |
| References |
| Boiling Point | 659.7ºC at 760 mmHg |
|---|---|
| Melting Point | 169ºC |
| Molecular Formula | C26H33ClN2O5 |
| Molecular Weight | 489.004 |
| Flash Point | 352.7ºC |
| Exact Mass | 488.207794 |
| PSA | 95.94000 |
| LogP | 3.80250 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~92% 
delapril hcl CAS#:83435-67-0 |
| Literature: Takeda Chemical Industries, Ltd. Patent: US4835302 A1, 1989 ; |
|
~91% 
delapril hcl CAS#:83435-67-0 |
| Literature: DIPHARMA FRANCIS s.r.l. Patent: US2008/15383 A1, 2008 ; Location in patent: Page/Page column 3 ; |
|
~71% 
delapril hcl CAS#:83435-67-0 |
| Literature: Miyake; Itoh; Oka Chemical and Pharmaceutical Bulletin, 1986 , vol. 34, # 7 p. 2852 - 2858 |
|
~% 
delapril hcl CAS#:83435-67-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 34, # 7 p. 2852 - 2858 |
|
~% 
delapril hcl CAS#:83435-67-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 34, # 7 p. 2852 - 2858 |
|
~% 
delapril hcl CAS#:83435-67-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 34, # 7 p. 2852 - 2858 |
|
~% 
delapril hcl CAS#:83435-67-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 34, # 7 p. 2852 - 2858 |
|
~% 
delapril hcl CAS#:83435-67-0 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 34, # 7 p. 2852 - 2858 |
| N-[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]-L-alanyl-N-(2,3-dihydro-1H-inden-2-yl)glycine hydrochloride (1:1) |
| MFCD00884619 |
| N-[(2S)-1-Ethoxy-1-oxo-4-phenyl-2-butanyl]-L-alanyl-N-(2,3-dihydro-1H-inden-2-yl)glycine hydrochloride (1:1) |
| delapril hcl |
| Glycine, N-[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanyl-N-(2,3-dihydro-1H-inden-2-yl)-, hydrochloride (1:1) |
![(S)-2-[(S)-1-[N-[(tert-Butoxycarbonyl)methyl]-N-(indan-2-yl)aminocarbonyl]ethylamino]-4-phenylbutyric acid ethyl ester structure](https://www.chemsrc.com/caspic/088/83435-61-4.png)
![Ethyl (S)-2-[(S)-4-methyl-2,5-dioxo-1,3-oxazolidin-3-yl]-4-phenylbutyrate structure](https://www.chemsrc.com/caspic/045/84793-24-8.png)
![N-[N-[(S)-1-ethoxycarbonyl-3-phenylpropyl]-L-alanyl]-N-(indan-2-yl)glycine structure](https://www.chemsrc.com/caspic/050/83435-62-5.png)
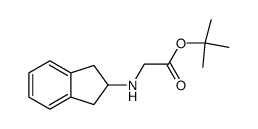
![N-[(S)-1-(ethoxycarbonyl)-3-phenylpropyl]-L-alanine tert-butylester structure](https://www.chemsrc.com/caspic/053/80828-38-2.png)
![Benzenebutanoicacid,-[[(1S)-1-carboxyethyl]amino]-,monoethylester,hydrochloride,(S)-(9CI) structure](https://www.chemsrc.com/caspic/346/80828-26-8.png)