Sultamicillin
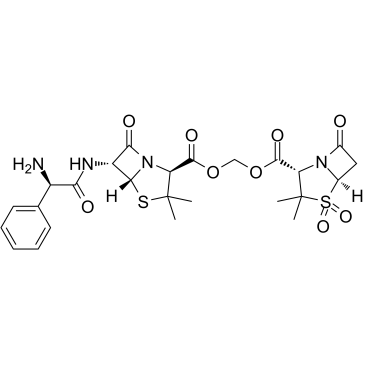
Sultamicillin structure
|
Common Name | Sultamicillin | ||
|---|---|---|---|---|
| CAS Number | 76497-13-7 | Molecular Weight | 594.657 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 907.7±65.0 °C at 760 mmHg | |
| Molecular Formula | C25H30N4O9S2 | Melting Point | 190° | |
| MSDS | N/A | Flash Point | 502.8±34.3 °C | |
Use of SultamicillinSultamicillin is an orally active double prodrug of Ampicillin/Sulbactan. Sulbactam is a semisynthetic beta-lactamase inhibitor which, in combination with Ampicillin, extends the antibacterial activity of the latter to include some beta-lactamase-producing strains of bacteria that would otherwise be resistant[1]. |
| Name | sultamicillin |
|---|---|
| Synonym | More Synonyms |
| Description | Sultamicillin is an orally active double prodrug of Ampicillin/Sulbactan. Sulbactam is a semisynthetic beta-lactamase inhibitor which, in combination with Ampicillin, extends the antibacterial activity of the latter to include some beta-lactamase-producing strains of bacteria that would otherwise be resistant[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 907.7±65.0 °C at 760 mmHg |
| Melting Point | 190° |
| Molecular Formula | C25H30N4O9S2 |
| Molecular Weight | 594.657 |
| Flash Point | 502.8±34.3 °C |
| Exact Mass | 594.145447 |
| PSA | 216.16000 |
| LogP | -0.29 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.668 |
| Storage condition | 2-8°C |
| Water Solubility | Practically insoluble in water, very slightly soluble in methanol, practically insoluble in ethanol (96 per cent). |
| HS Code | 2934999090 |
|---|
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Clinical response at Day 3 of therapy and economic outcomes in hospitalized patients with acute bacterial skin and skin structure infection (ABSSSI).
Curr. Med. Res. Opin. 29(7) , 869-77, (2013) The FDA recently issued guidance for the types of infections that should be included in trials to support an indication for antibacterial treatment. The latest FDA guidance recommends assessing respon... |
|
|
Antibiotic regimens for management of intra-amniotic infection.
Cochrane Database Syst. Rev. 12 , CD010976, (2014) Chorioamnionitis is a common infection that affects both mother and infant. Infant complications associated with chorioamnionitis include early neonatal sepsis, pneumonia, and meningitis. Chorioamnion... |
|
|
Anaerobiospirillum succiniciproducens-induced bacteremia in a healthy man.
Am. J. Emerg. Med. 32(7) , 812.e1-3, (2014) Anaerobiospirillum succiniciproducens is rarely associated with bacteremia but results in significant mortality. Almost all reported bacteremia cases have occurred in immunocompromised hosts, such as ... |
| 6'-(2-Amino-2-phenylacetamido)penicillanoyloxymethylpenicillanate 1,1-dioxide |
| Sultancillin alkali |
| 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-7-oxo-, [[[(2S,5R,6R)-6-[[(2R)-2-amino-2-phenylacetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-yl]carbonyl]oxy]methyl ester, 4,4-dioxide, (2S,5R)- |
| Unacid PD |
| SultaMcillin Base |
| VD 1827 |
| 1,1-Dioxopenicillanoyloxymethyl 6-(D-a-amino-a-phenylacetamido)penicillanate |
| ({[(2S,5R,6R)-6-{[(2R)-2-Amino-2-phenylacetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-yl]carbonyl}oxy)methyl (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-dioxide |
| Sultamicillin |
| Bethadl Orale |
| Sultamicillin Base |
| Baeimex |
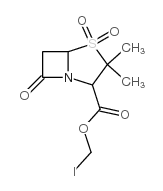 CAS#:76247-39-7
CAS#:76247-39-7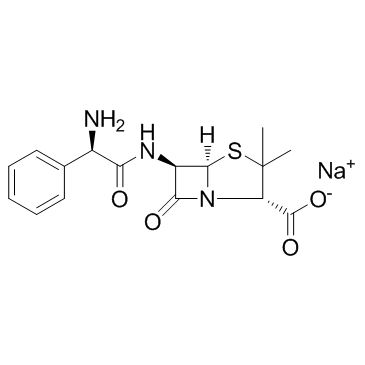 CAS#:69-52-3
CAS#:69-52-3