a-Isothiocyanatotoluene
Modify Date: 2024-01-02 21:09:10
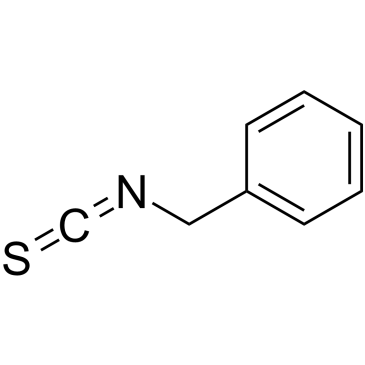
a-Isothiocyanatotoluene structure
|
Common Name | a-Isothiocyanatotoluene | ||
|---|---|---|---|---|
| CAS Number | 622-78-6 | Molecular Weight | 149.213 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 243.8±9.0 °C at 760 mmHg | |
| Molecular Formula | C8H7NS | Melting Point | 41 °C | |
| MSDS | Chinese USA | Flash Point | 100.4±26.5 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of a-IsothiocyanatotolueneBenzyl isothiocyanate is a member of natural isothiocyanates with antimicrobial activity[1][2]. Benzyl isothiocyanate potent inhibits cell mobility, migration and invasion nature and matrix metalloproteinase-2 (MMP-2) activity of murine melanoma cells[2]. |
| Name | benzyl isothiocyanate |
|---|---|
| Synonym | More Synonyms |
| Description | Benzyl isothiocyanate is a member of natural isothiocyanates with antimicrobial activity[1][2]. Benzyl isothiocyanate potent inhibits cell mobility, migration and invasion nature and matrix metalloproteinase-2 (MMP-2) activity of murine melanoma cells[2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 243.8±9.0 °C at 760 mmHg |
| Melting Point | 41 °C |
| Molecular Formula | C8H7NS |
| Molecular Weight | 149.213 |
| Flash Point | 100.4±26.5 °C |
| Exact Mass | 149.029922 |
| PSA | 44.45000 |
| LogP | 3.02 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.560 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H312-H315-H319-H332-H334-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338-P342 + P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | 2810 |
| WGK Germany | 3 |
| RTECS | NX8250000 |
| Packaging Group | III |
| Hazard Class | 8 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
|
In vitro metabolic conversion of the organic breakdown products of glucosinolate to goitrogenic thiocyanate anion.
J. Sci. Food Agric. 95 , 2244-51, (2015) Glucosinolates are abundant in Brassicaceae vegetables, and they are degraded into various organic breakdown products (BPs) (R-CN, -NCS and -SCN) by myrosinase when plant tissues are damaged. This stu... |
|
|
Cytochrome p450 enzymes mechanism based inhibitors: common sub-structures and reactivity.
Curr. Drug Metab. 6 , 413-54, (2005) The inhibition of human cytochrome P450s (CYPs) is one of the most common mechanisms which can lead to drug-drug interactions. The inhibition of CYPs can be reversible (competitive or non-competitive)... |
|
|
Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents.
J. Med. Chem. 53 , 5085-107, (2010)
|
| MFCD00004819 |
| Toluene, α-isothiocyanato- |
| (Isothiocyanatomethyl)benzene |
| a-Isothiocyanatotoluene |
| EINECS 210-753-9 |
| Benzyl isothiocyanate |
| Benzene, (isothiocyanatomethyl)- |
 CAS#:701-72-4
CAS#:701-72-4 CAS#:333-20-0
CAS#:333-20-0 CAS#:100-44-7
CAS#:100-44-7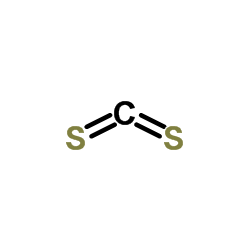 CAS#:75-15-0
CAS#:75-15-0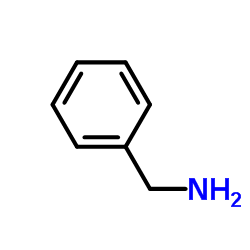 CAS#:100-46-9
CAS#:100-46-9 CAS#:463-71-8
CAS#:463-71-8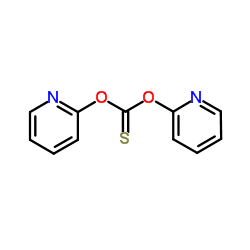 CAS#:96989-50-3
CAS#:96989-50-3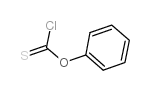 CAS#:1005-56-7
CAS#:1005-56-7![S-[N-BENZYL(THIOCARBAMOYL)]-L-CYSTEINE structure](https://www.chemsrc.com/caspic/454/35446-36-7.png) CAS#:35446-36-7
CAS#:35446-36-7 CAS#:35653-54-4
CAS#:35653-54-4 CAS#:4238-71-5
CAS#:4238-71-5![4-benzyl-2-tert-butyl-3-phenyl-[1,2,4]oxadiazolidine-5-thione structure](https://www.chemsrc.com/caspic/146/72995-56-3.png) CAS#:72995-56-3
CAS#:72995-56-3![n-tert-butyl-n-[(e)-phenylmethylene]amine oxide structure](https://www.chemsrc.com/caspic/242/3376-24-7.png) CAS#:3376-24-7
CAS#:3376-24-7 CAS#:226908-20-9
CAS#:226908-20-9 CAS#:18720-46-2
CAS#:18720-46-2 CAS#:21076-23-3
CAS#:21076-23-3![8-benzyl-10-oxa-8-azabicyclo[4.4.0]deca-1,3,5-triene-7,9-dione structure](https://www.chemsrc.com/caspic/056/2037-99-2.png) CAS#:2037-99-2
CAS#:2037-99-2
