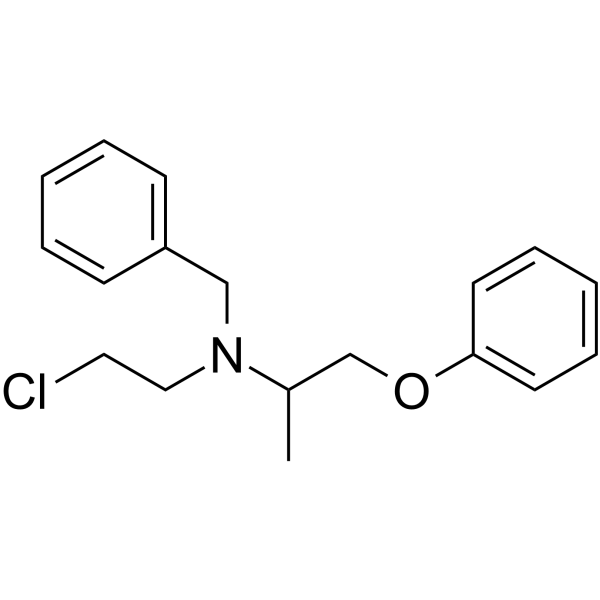
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
DP3500000
-
CHEMICAL NAME :
-
Benzylamine, N-(2-chloroethyl)-N-(1-methyl-2-phenoxyethyl)-
-
CAS REGISTRY NUMBER :
-
59-96-1
-
BEILSTEIN REFERENCE NO. :
-
2129697
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
16
-
MOLECULAR FORMULA :
-
C18-H22-Cl-N-O
-
MOLECULAR WEIGHT :
-
303.86
-
WISWESSER LINE NOTATION :
-
G2N1R&Y1&1OR
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
27ZIAQ "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973 Volume(issue)/page/year: -,195,1973
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intracerebral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3400 ug/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - altered sleep time (including change in righting reflex) Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
AITEAT Archivum Immunologiae et Therapiae Experimentalis. (Ars Polona, POB 1001, 00-068 Warsaw 1, Poland) V.10- 1962- Volume(issue)/page/year: 24,223,1976
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1535 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
27ZIAQ "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973 Volume(issue)/page/year: -,195,1973
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
10 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
27ZIAQ "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973 Volume(issue)/page/year: -,195,1973
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
27ZIAQ "Drug Dosages in Laboratory Animals - A Handbook," Rev. ed., Barnes, C.D., and L.G. Eltherington, Berkeley, Univ. of California Press, 1973 Volume(issue)/page/year: -,195,1973 ** TUMORIGENIC DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
100 mg/kg/8W-I
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Lungs, Thorax, or Respiration - tumors
-
REFERENCE :
-
CNREA8 Cancer Research. (Public Ledger Building, Suit 816, 6th & Chestnut Sts., Philadelphia, PA 19106) V.1- 1941- Volume(issue)/page/year: 33,3069,1973 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2571 ug/kg
-
SEX/DURATION :
-
male 18 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 29,479,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
3 mg/kg
-
SEX/DURATION :
-
male 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - male fertility index (e.g. # males impregnating females per # males exposed to fertile nonpregnant females)
-
REFERENCE :
-
TOLED5 Toxicology Letters. (Elsevier Science Pub. B.V., POB 211, 1000 AE Amsterdam, Netherlands) V.1- 1977- Volume(issue)/page/year: 52,221,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
12 mg/kg
-
SEX/DURATION :
-
female 4-5 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina Reproductive - Maternal Effects - other effects
-
REFERENCE :
-
JRPFA4 Journal of Reproduction and Fertility. (Biochemical Soc. Book Depot, POB 32, Commerce Way, Colchester, Essex CO2 8HP, UK) V.1- 1960- Volume(issue)/page/year: 81,51,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
9 mg/kg
-
SEX/DURATION :
-
female 1-2 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
JRPFA4 Journal of Reproduction and Fertility. (Biochemical Soc. Book Depot, POB 32, Commerce Way, Colchester, Essex CO2 8HP, UK) V.1- 1960- Volume(issue)/page/year: 17,579,1968
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
39 mg/kg
-
SEX/DURATION :
-
female 2 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
JRPFA4 Journal of Reproduction and Fertility. (Biochemical Soc. Book Depot, POB 32, Commerce Way, Colchester, Essex CO2 8HP, UK) V.1- 1960- Volume(issue)/page/year: 35,331,1973
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravaginal
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated) Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
CCPTAY Contraception. (Geron-X, Inc., POB 1108, Los Altos, CA 94022) V.1- 1970- Volume(issue)/page/year: 17,309,1978 *** REVIEWS *** IARC Cancer Review:Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 9,223,1975 IARC Cancer Review:Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 24,185,1980 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X7152 No. of Facilities: 9 (estimated) No. of Industries: 1 No. of Occupations: 1 No. of Employees: 339 (estimated) No. of Female Employees: 170 (estimated)
|