Withaferin A
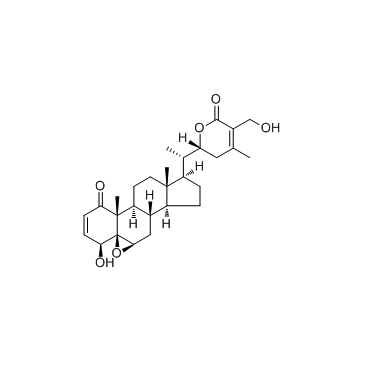
Withaferin A structure
|
Common Name | Withaferin A | ||
|---|---|---|---|---|
| CAS Number | 5119-48-2 | Molecular Weight | 470.598 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 680.7±55.0 °C at 760 mmHg | |
| Molecular Formula | C28H38O6 | Melting Point | 252-253ºC | |
| MSDS | Chinese USA | Flash Point | 226.7±25.0 °C | |
Use of Withaferin AWithaferin A is a steroidal lactone isolated from Withania somnifera, inhibits NF-kB activation and targets vimentin, with potent antiinflammatory and anticancer activities. |
| Name | withaferin A |
|---|---|
| Synonym | More Synonyms |
| Description | Withaferin A is a steroidal lactone isolated from Withania somnifera, inhibits NF-kB activation and targets vimentin, with potent antiinflammatory and anticancer activities. |
|---|---|
| Related Catalog | |
| Target |
NFκB |
| In Vitro | Withaferin A has antiinflammatory activity, and potently inhibits NF-kB activation by preventing the TNF-induced activation of Ik-B kinase beta via a thioalkylation-sensitive redox mechanism[1]. Withaferin A also has anticancer activity. Withaferin A targets the IF protein vimentin, causes aggregation of vimentin filaments in bovine aortic endothelial cells (BAECs) at 3 μM, and induces vimentin fragmentation in endothelial cells at 10 μM[2]. Withaferin A (0.5, 1.5 μM) alone or incombination with cisplatin (CIS) dose-dependently reduces tumorigenic potential of ALDH1 positive cancer stem cells (CSCs)[3]. |
| In Vivo | Withaferin A (2 mg/kg, i.p.) shows potent angiogenesis inhibitory activity via vimentin in mice[2]. Withaferin A (2 mg/kg) combined with cisplatin (CIS) regulates the expression of ALDH1 marker, and downregulates the expression of securin in tumors collected from mice[3]. |
| Cell Assay | Ovarian epithelial cancer cell line A2780 is maintained in RPMI1640 medium supplemented with insulin (5 μg/mL), penicillin/streptomycin (100 IU/mL and 100 μg/mL respectively) and 10% fetal bovine serum (FBS) from Hyclone. Withaferin A, cisplatin (CIS) and other reagents are prepared in DMSO. Cisplatin is prepared fresh each time[3]. |
| Animal Admin | Mice[2] Vimentin homozygous-deficient mice (Vim−/−) and mice that are vimentin-heterozygous deficient (Vim+/−) in the 129/Svev background are used in the assay. In brief, mice between 4 and 6 wk of age are anesthetized by intraperiteoneal (i.p.) injection of ketamine and xylazine. Corneas are topically anesthetized by application of proparacain eye drop, and 1 μL drop of dilute 0.15 M sodium hydroxide is applied for 1 min. The cornea is immediately washed extensively in saline solution, and corneal and limbal epithelium gently removed by scraping. The cornea is topically treated with atropine eye drop and covered with tobramycin and erythromycin antibiotic eye ointment. Withaferin A or 12-D WS (2 mg/kg solubilized in DMSO) or vehicle (DMSO) is injected i.p. in respective drug or control groups of mice after their recovery from corneal injury, and subsequently every day thereafter for a period of 10 days. Mice are humanely killed and eyes enucleated. The anterior segment half of eyes are dissected and corneal buttons are prepared. Corneal tissues are fixed in 100% acetone for 20 min, washed in PBS for 1 hr, and blocked for 18 hr in 1% BSA-PBS at 4°C. Cornea whole-mount staining is performed by incubating tissues in FITC-conjugated rat anti-mouse CD31 antibody (1:333 dilution in 1% BSA-PBS) for 12 hr, washed away for 24 hr at 4°C in 1% BSA-PBS, and affixed to glass slides with a coverslip. Fluorescent staining is visualized on microscope, and quantified by importing digital images to NIH ImageJ[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 680.7±55.0 °C at 760 mmHg |
| Melting Point | 252-253ºC |
| Molecular Formula | C28H38O6 |
| Molecular Weight | 470.598 |
| Flash Point | 226.7±25.0 °C |
| Exact Mass | 470.266846 |
| PSA | 96.36000 |
| LogP | 3.80 |
| Vapour Pressure | 0.0±4.8 mmHg at 25°C |
| Index of Refraction | 1.599 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | T |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | KE7288500 |
|
~% 
Withaferin A CAS#:5119-48-2 |
| Literature: Hirayama; Gamoh; Ikekawa Tetrahedron Letters, 1982 , vol. 23, # 45 p. 4725 - 4728 |
|
~% 
Withaferin A CAS#:5119-48-2 |
| Literature: Hirayama; Gamoh; Ikekawa Tetrahedron Letters, 1982 , vol. 23, # 45 p. 4725 - 4728 |
|
~% 
Withaferin A CAS#:5119-48-2 |
| Literature: Hirayama; Gamoh; Ikekawa Tetrahedron Letters, 1982 , vol. 23, # 45 p. 4725 - 4728 |
|
~% 
Withaferin A CAS#:5119-48-2 |
| Literature: Hirayama; Gamoh; Ikekawa Tetrahedron Letters, 1982 , vol. 23, # 45 p. 4725 - 4728 |
|
~% 
Withaferin A CAS#:5119-48-2 |
| Literature: Hirayama; Gamoh; Ikekawa Tetrahedron Letters, 1982 , vol. 23, # 45 p. 4725 - 4728 |
|
~% 
Withaferin A CAS#:5119-48-2 |
| Literature: Hirayama; Gamoh; Ikekawa Tetrahedron Letters, 1982 , vol. 23, # 45 p. 4725 - 4728 |
|
Multi-layer polymeric implants for sustained release of chemopreventives.
Cancer Lett. 326(1) , 33-40, (2012) Poor oral bioavailability limits the use of many chemopreventives in the prevention and treatment of cancer. To overcome this limitation, we report an improvised implant formulation ("coated" implants... |
|
|
Withaferin A suppresses tumor promoter 12-O-tetradecanoylphorbol 13-acetate-induced decreases in isocitrate dehydrogenase 1 activity and mitochondrial function in skin epidermal JB6 cells.
Cancer Sci. 104(2) , 143-8, (2013) Withaferin A (WA) is a bioactive compound derived from Withania somnifera. The antitumor activity of WA has been well studied in human cancer models; however, its chemopreventive potential is unclear.... |
|
|
Withaferin A induces proteasome inhibition, endoplasmic reticulum stress, the heat shock response and acquisition of thermotolerance.
PLoS ONE 7(11) , e50547, (2012) In the present study, withaferin A (WA), a steroidal lactone with anti-inflammatory and anti-tumor properties, inhibited proteasome activity and induced endoplasmic reticulum (ER) and cytoplasmic HSP ... |
| Withaferin A |
| (4β,5β,6β,22R)-4,27-Dihydroxy-5,6:22,26-diepoxyergosta-2,24-diene-1,26-dione |
| 5,6-Epoxy-4,22,27-trihydroxy-1-oxoergosta-2,24-dien-26-oic Acid d-Lactone |
| Ergosta-2,24-diene-1,26-dione, 5,6:22,26-diepoxy-4,27-dihydroxy-, (4β,5β,6β,22R)- |
| (4b,5b,6b,22R)-5,6-Epoxy-4,22,27-trihydroxy-1-oxoergosta-2,24-dien-26-oic acid d-lactone |
| 4b,27-Dihydroxy-1-oxo-5b,6b-epoxywitha-2,24-dienolide |
| 5,6-Epoxy-4,27-dihydroxy-1-oxowitha-2,24-dienolide,Withaferine A |
![(6R)-6-((1S)-1-((4S,8S,9S,10R,13S,14S)-4-hydroxy-10,13-dimethyl-1-oxo-4,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethyl)-3-(hydroxymethyl)-4-methyl-5,6-dihydro-2H-pyran-2-one structure](https://image.chemsrc.com/caspic/399/81345-21-3.png)
![(6R)-6-((1S)-1-((1S,3R,8S,9S,10R,13S,14S)-3-((tert-butyldimethylsilyl)oxy)-1-hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethyl)-3-(hydroxymethyl)-4-methyl-5,6-dihydro-2H-pyran-2-one structure](https://image.chemsrc.com/caspic/416/81874-42-2.png)
![(6R)-6-((1S)-1-((6R,8S,9S,10R,13S,14S)-10,13-dimethyl-1-oxo-6-(phenylthio)-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethyl)-3-(hydroxymethyl)-4-methyl-5,6-dihydro-2H-pyran-2-one structure](https://image.chemsrc.com/caspic/071/81426-86-0.png)
![(6R)-6-((1S)-1-((1S,3S,4aR,5aS,6aS,6bS,9aS,11aS,11bS)-3-((tert-butyldimethylsilyl)oxy)-1-hydroxy-9a,11b-dimethylhexadecahydrocyclopenta[1,2]phenanthro[8a,9-b]oxiren-9-yl)ethyl)-3-(((tert-butyldimethylsilyl)oxy)methyl)-4-methyl-5,6-dihydro-2H-pyran-2-one structure](https://image.chemsrc.com/caspic/157/81426-84-8.png)
![(3R,4aR,5aS,6aS,6bS,9aS,11aS,11bR)-3-((tert-butyldimethylsilyl)oxy)-9-((S)-1-((R)-5-(((tert-butyldimethylsilyl)oxy)methyl)-4-methyl-6-oxo-3,6-dihydro-2H-pyran-2-yl)ethyl)-9a,11b-dimethyltetradecahydrocyclopenta[1,2]phenanthro[8a,9-b]oxiren-1(2H)-one structure](https://image.chemsrc.com/caspic/431/85427-87-8.png)
![(6R)-6-((1S)-1-((3R,5R,6R,8S,9S,10R,13S,14S)-3-((tert-butyldimethylsilyl)oxy)-5-hydroxy-10,13-dimethyl-1-oxo-6-(phenylthio)hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethyl)-3-(((tert-butyldimethylsilyl)oxy)methyl)-4-methyl-5,6-dihydro-2H-pyran-2-one structure](https://image.chemsrc.com/caspic/119/81426-85-9.png)
 CAS#:1214886-35-7
CAS#:1214886-35-7 CAS#:22848-79-9
CAS#:22848-79-9
