Levalbuterol Hydrochloride
Modify Date: 2024-01-03 18:32:16
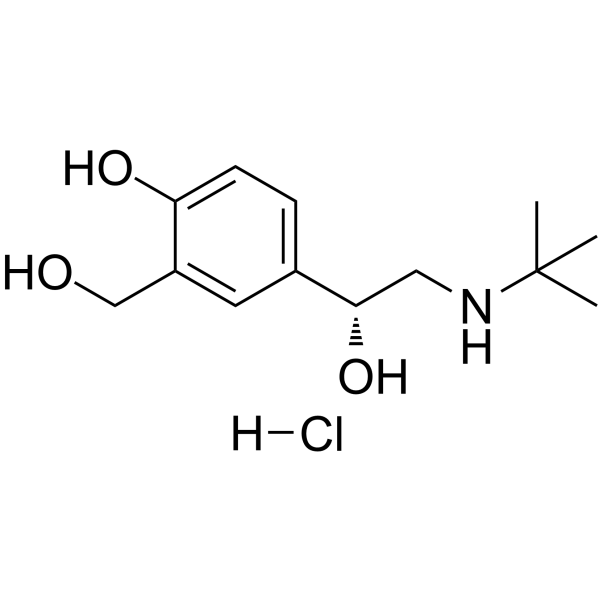
Levalbuterol Hydrochloride structure
|
Common Name | Levalbuterol Hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 50293-90-8 | Molecular Weight | 275.772 | |
| Density | N/A | Boiling Point | 433.5ºC at 760 mmHg | |
| Molecular Formula | C13H22ClNO3 | Melting Point | 169-171ºC | |
| MSDS | USA | Flash Point | N/A | |
| Symbol |

GHS05, GHS07 |
Signal Word | Danger | |
Use of Levalbuterol HydrochlorideLevalbuterol ((R)-Albuterol) hydrochloride is a short-acting β2-adrenergic receptor agonist and the active (R)-enantiomer of Salbutamol. Levalbuterol hydrochloride is a more potent bronchodilator than Salbutamol and has the potential for the treatment of COPD[1]. |
| Name | 2-(Hydroxymethyl)-4-{(1R)-1-hydroxy-2-[(2-methyl-2-propanyl)amino ]ethyl}phenol hydrochloride (1:1) |
|---|---|
| Synonym | More Synonyms |
| Description | Levalbuterol ((R)-Albuterol) hydrochloride is a short-acting β2-adrenergic receptor agonist and the active (R)-enantiomer of Salbutamol. Levalbuterol hydrochloride is a more potent bronchodilator than Salbutamol and has the potential for the treatment of COPD[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Levalbuterol (10 μM; 24 hours) hydrochloride induces 11β-HSD1 mRNA expression, however, it does not influence 11β-HSD2expression in airway epithelial cells[1]. Levalbuterol (10 μM; 24 hours) hydrochloride significantly reduces both LPS- and TNF-α-induced NF-κB activity while increasing GRE activation in an 11β-HSD1 dependent manner in a transformed mouse airway epithelial cell line[1]. RT-PCR[1] Cell Line: Murine Club (MTCC) cells Concentration: 10 μM Incubation Time: 24 hours Result: Increased 11β-HSD1 mRNA expression selectively. |
| In Vivo | Levalbuterol (subcutaneous injection; 1 mg/kg; 14 days) hydrochloride significantly decreases pulmonary inflammation in OVA mice, demonstrated a decrease in eosinophilia and IgE[2]. Animal Model: C57BL/6 female mice with a pulmonary allergic model[2] Dosage: 1 mg/kg Administration: Subcutaneous injection; 1 mg/kg; 14 days Result: Decreased pulmonary inflammation after OVA sensitization. |
| References |
| Boiling Point | 433.5ºC at 760 mmHg |
|---|---|
| Melting Point | 169-171ºC |
| Molecular Formula | C13H22ClNO3 |
| Molecular Weight | 275.772 |
| Exact Mass | 275.128815 |
| PSA | 72.72000 |
| LogP | 2.49890 |
| Storage condition | -20°C |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H318-H335 |
| Precautionary Statements | P280-P301 + P312 + P330-P305 + P351 + P338 + P310 |
| Hazard Codes | Xn |
| Risk Phrases | 22-37/38-41 |
| Safety Phrases | 26-39 |
| RIDADR | NONH for all modes of transport |
|
Levalbuterol versus racemic albuterol in the treatment of acute exacerbation of asthma in children.
Pediatr. Emerg. Care 21(7) , 415-9, (2005) To compare levalbuterol and racemic albuterol for the treatment of acute exacerbation of asthma in pediatric population.Prospective, double-blind, randomized research trial in a pediatric emergency de... |
| MFCD08067728 |
| (R)-Albuterol hydrochloride |
| levosalbutamol hydrochloride |
| Levalbuterol Hydrochloride |
| (-)-a1-(((1,1-Dimethylethyl)amino)methyl)-4-hydroxy-1,3-benzenedimethanol Hydrochloride |
| 4-[(1R)-2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)phenol hydrochloride |
| 1,3-benzenedimethanol, α-[[(1,1-dimethylethyl)amino]methyl]-4-hydroxy-, (αR)-, hydrochloride |
| 1,3-Benzenedimethanol, α-[[(1,1-dimethylethyl)amino]methyl]-4-hydroxy-, (αR)-, hydrochloride (1:1) |
| (R)-Salbutamol hydrochloride |
| 2-(Hydroxymethyl)-4-{(1R)-1-hydroxy-2-[(2-methyl-2-propanyl)amino]ethyl}phenol hydrochloride (1:1) |
| Levalbuterol HCl |
| (-)-Salbutamol hydrochloride |
| (r)-(-)-a1-((tert-butylamino)methyl)-4-hydroxy-m-xylene-a,a'-diol hydrochloride |
| 2-(Hydroxymethyl)-4-{(1R)-1-hydroxy-2-[(2-methyl-2-propanyl)amino]ethyl}phenol hydrochloride |
| 4-[(1R)-2-(tert-Butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)phenol hydrochloride (1:1) |
| UNII-WDQ1526QJM |
| (a1R)-a1-[[(1,1-Dimethylethyl)amino]methyl]-4-hydroxy-1,3-benzenedimethanol Hydrochloride |
| Xopenex |
| R-albuterol hydrochloride |
| (R)-salbutamol hydrochloride Levosalbutamol hydrochloride |