JNJ-7706621
Modify Date: 2024-01-09 11:09:31
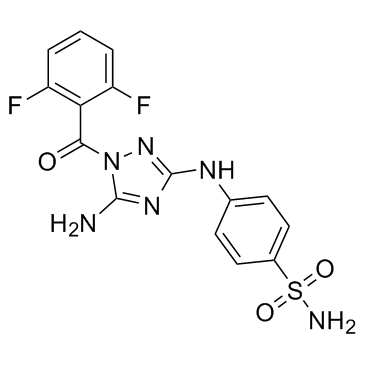
JNJ-7706621 structure
|
Common Name | JNJ-7706621 | ||
|---|---|---|---|---|
| CAS Number | 443797-96-4 | Molecular Weight | 394.356 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 676.6±65.0 °C at 760 mmHg | |
| Molecular Formula | C15H12F2N6O3S | Melting Point | 149-155ºC | |
| MSDS | N/A | Flash Point | 363.0±34.3 °C | |
Use of JNJ-7706621JNJ-7706621 is a potent aurora kinase inhibitor, and also inhibits CDK1 and CDK2, with IC50s of 9, 3, 11, and 15 nM for CDK1, CDK2, Aurora-A and Aurora-B, respectively. |
| Name | 4-[[5-amino-1-(2,6-difluorobenzoyl)-1,2,4-triazol-3-yl]amino]benzenesulfonamide |
|---|---|
| Synonym | More Synonyms |
| Description | JNJ-7706621 is a potent aurora kinase inhibitor, and also inhibits CDK1 and CDK2, with IC50s of 9, 3, 11, and 15 nM for CDK1, CDK2, Aurora-A and Aurora-B, respectively. |
|---|---|
| Related Catalog | |
| Target |
CDK2/cyclinE:3 nM (IC50) cdk2/cyclin A:4 nM (IC50) Cdk1/cyclin B:9 nM (IC50) CDK3/Cyclin E:58 nM (IC50) CDK6/cyclinD1:175 nM (IC50) Cdk4/cyclin D1:253 nM (IC50) Aurora A:11 nM (IC50) Aurora B:15 nM (IC50) VEGF-R1:6400 nM (IC50) VEGF-R2:154 nM (IC50) VEGF-R3:735 nM (IC50) FGF-R1:575 nM (IC50) FGF-R2:226 nM (IC50) GSK3β:254 nM (IC50) |
| In Vitro | JNJ-7706621 shows antiproliferative activity against various human tumor cells with IC50s of 284, 254, and 447 nM for HeLa, HCT116, and A375, respectively[1]. JNJ-7706621 inhibits other centrosomal proteins such as TOG, Nek2, and TACC3 in early mitotic phase, but does not prevent localization of Aurora A to the spindle poles. Treatment of nocodazole-synchronized cells with JNJ-7706621 can override mitotic arrest by preventing spindle checkpoint signaling, resulting in failure of chromosome alignment and segregation[2]. JNJ-7706621 suspensions inhibits cell viability of HeLa cells with IC50s of 2.1 and 0.9 μg/mL at 24 and 48 h. The IC50 of the JNJ-7706621-loaded nanoparticles are 35 and 2.7 μg/mL and the IC50 of the JNJ-7706621-loaded micelles are 6.3 and 1.6 μg/mL[3]. JNJ-7706621 shows inhibition of Aurora-A and Aurora-B but has no activity at the highest concentration tested on the Plk1 or Wee1 serine/threonine kinases. JNJ-7706621 also shows potent growth inhibition in vitro on all human cancer cell types with IC50 values ranging from 112 to 514 nM[4]. |
| In Vivo | JNJ-7706621 (100 mg/kg, i.p.) exhibits 95% tumor growth inhibition in A375 (human melanoma) tumor xenograft model[1]. JNJ-7706621-loaded micelles inhibit tumor growth, and delay the tumor growth more efficiently than the control JNJ-7706621 suspension[3]. JNJ-7706621 (100 and 125 mg/kg) is efficacious in a human tumor xenograft model under intermittent dosing regimens[4]. |
| Kinase Assay | To identify compounds that inhibit CDK1 kinase activity, a screening method is developed using the CDK1/cyclin B complex to phosphorylate a biotinylated peptide substrate containing the consensus phosphorylation site for histone H1, which is phosphorylatedin vivo by CDK1. Inhibition of CDK1 activity is measured by observing a reduced amount of 33P-g-ATP incorporation into the immobilized substrate in streptavidin-coated 96-well scintillating microplates. CDK1 enzyme is diluted in 50 mM Tris-HCl (pH 8), 10 mM MgCl2, 0.1 mM Na3VO4, 1 mM DTT, 1% DMSO, 0.25 AM peptide, 0.1 ACi per well 33P-g-ATP (2,000-3,000 Ci/mmol), and 5 AM ATP in the presence or absence of various concentrations of test compound and incubated at 30°C for 1 hour. The reaction is terminated by washing with PBS containing 100 mM EDTA and plates are counted in a scintillation counter. Linear regression analysis of the percent inhibition by test compound is used to determine IC50 values. The Aurora kinase assays are done with 10 AM ATP and a peptide containing a dual repeat of the kemptide phosphorylation motif. |
| Cell Assay | HeLa cells are seeded in 96-well plates at the density of 2500 viable cells per well. The cells are then incubated with a suspension of JNJ-7706621, JNJ-7706621-loaded micelles and nanoparticles (JNJ-7706621 concentrations of 0.011, 0.022, 0.11, 0.22, 1.1, 2.2, 11 and 22 μg/mL; dilutions are made in the medium) and drug-free polymeric micelles (polymers concentrations 0.3 mg/mL) and nanoparticles (polymers concentration 5 mg/mL) for 4, 24 and 48 h. The cytotoxicity is assessed using the MTT test. Absorbance is measured at 570 nm using a microplate reader. Untreated cells are taken as control with 100% viability and Triton X-100 1% is used as positive control of cytotoxicity. The results are expressed as mean values ± standard deviations of five measurements. |
| Animal Admin | Briefly, animals are implanted s.c. with 1 mm3 A375 tumor fragments in the hindflank. After tumors reach 62 to 126 mg, groups are pair matched. Animals are given JNJ-7706621 or vehicle control starting on day 1. The tumor growth delay method is followed where each animal is euthanized when its neoplasm reached a predetermined size of 2.0 g. All statistical analyses are conducted using unpaired t tests at a P level of 0.05 (two tailed). |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 676.6±65.0 °C at 760 mmHg |
| Melting Point | 149-155ºC |
| Molecular Formula | C15H12F2N6O3S |
| Molecular Weight | 394.356 |
| Flash Point | 363.0±34.3 °C |
| Exact Mass | 394.065979 |
| PSA | 154.37000 |
| LogP | 0.18 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.724 |
| Storage condition | -20℃ |
| Water Solubility | Soluble in DMSO at 15mg/ml |
| HS Code | 2935009090 |
|---|
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
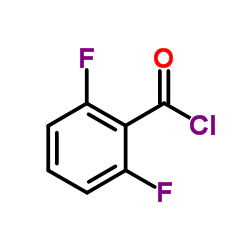
| 4-{[5-Amino-1-(2,6-difluorobenzoyl)-1H-1,2,4-triazol-3-yl]amino}benzenesulfonamide |
| Benzenesulfonamide, 4-[[5-amino-1-(2,6-difluorobenzoyl)-1H-1,2,4-triazol-3-yl]amino]- |
| 3ama |
| JNJ-7706621 |
![N-[4-(aminosulfonyl)phenyl]-N'-cyanocarbamidic acid phenyl ester structure](https://www.chemsrc.com/caspic/244/700805-72-7.png)
![4-(5-amino-1H-[1,2,4]triazol-3-ylamino)benzenesulfonamide structure](https://www.chemsrc.com/caspic/385/443799-31-3.png)