H-Pro-NH2.HCl
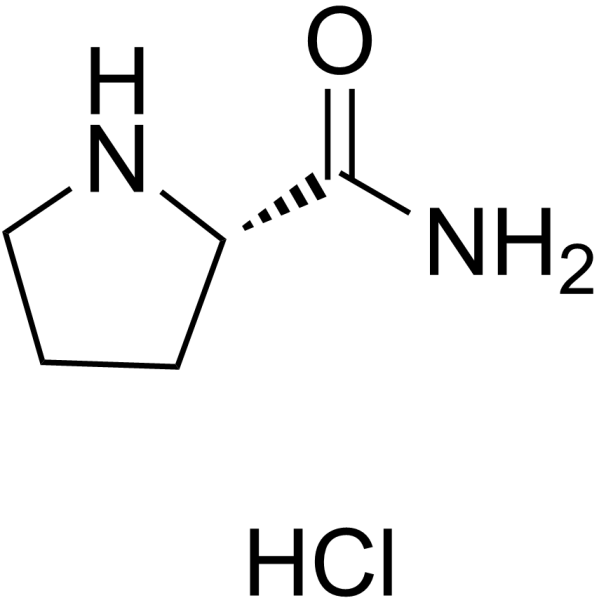
H-Pro-NH2.HCl structure
|
Common Name | H-Pro-NH2.HCl | ||
|---|---|---|---|---|
| CAS Number | 42429-27-6 | Molecular Weight | 150.607 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C5H11ClN2O | Melting Point | 178-182 ℃ | |
| MSDS | USA | Flash Point | N/A | |
Use of H-Pro-NH2.HCl(S)-Pyrrolidine-2-carboxamide hydrochloride is a proline derivative[1]. |
| Name | (2S)-pyrrolidine-2-carboxamide,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | (S)-Pyrrolidine-2-carboxamide hydrochloride is a proline derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Melting Point | 178-182 ℃ |
|---|---|
| Molecular Formula | C5H11ClN2O |
| Molecular Weight | 150.607 |
| Exact Mass | 150.055984 |
| PSA | 55.12000 |
| LogP | 1.05480 |
| Storage condition | -15°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Enantioseparation of amino acids and alpha-hydroxy acids on ligand-exchange continuous beds by capillary electrochromatography.
Electrophoresis 31 , 1517-1520, (2010) A new chiral stationary phase based on continuous bed (CB) technology using L-prolinamide as a chiral selector was prepared. Its ability for enantioseparation of amino acids and alpha-hydroxy acids by... |
|
|
Enantioseparation by chromatographic and electromigration techniques using ligand-exchange as chiral separation principle.
Anal. Bioanal. Chem 400 , 2305-2316, (2011) This article gives a short overview of the application of the principle of chiral ligand-exchange in HPLC, CE, and CEC. Since its introduction by Davankov, more than thousand articles have appeared in... |
|
|
Chiral separation of NBD-amino acids by ligand-exchange micro-channel electrophoresis.
Anal. Sci. 21 , 67-71, (2005) The chiral separation of amino acid derivatives by ligand-exchange electrophoresis in a microchannel chip was performed for the first time. A Cu(II) complex with L-prolinamide was used as a chiral sel... |
| EINECS 255-818-2 |
| L-Proline amide hydrochloride |
| HCl*H-L-Pro-NH2 |
| L-Prolinamidehydrochloride |
| Prolinamide hydrochloride (1:1) |
| 2-Pyrrolidinecarboxamide, (2S)-, hydrochloride (1:1) |
| dl-prolinamide hydrochloride |
| MFCD00058156 |
| Pro-NH2*HCl |
| 2-Pyrrolidinecarboxamide, hydrochloride (1:1) |
| L-Prolinamide hydrochloride (1:1) |
| L-Prolinamide HCl |
| H-Pro-NH2.HCl |
 CAS#:35150-07-3
CAS#:35150-07-3 CAS#:3392-10-7
CAS#:3392-10-7 CAS#:2133-40-6
CAS#:2133-40-6 CAS#:76409-77-3
CAS#:76409-77-3![tert-butyl 5-[(2R)-3-[(2S)-2-carbamoylpyrrolidin-1-yl]-2-[(2-methylpropan-2-yl)oxycarbonylamino]-3-oxopropyl]imidazole-1-carboxylate structure](https://www.chemsrc.com/caspic/122/50654-97-2.png) CAS#:50654-97-2
CAS#:50654-97-2 CAS#:29133-55-9
CAS#:29133-55-9 CAS#:147-85-3
CAS#:147-85-3
