S-(2-Aminoethyl)-L-cysteine hydrochloride
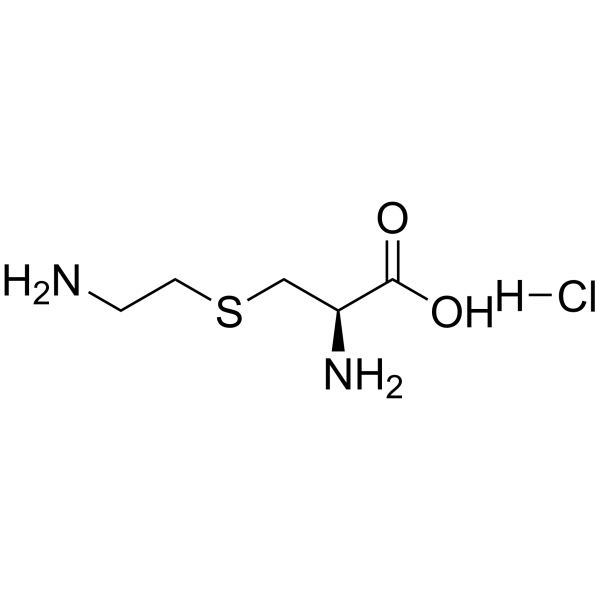
S-(2-Aminoethyl)-L-cysteine hydrochloride structure
|
Common Name | S-(2-Aminoethyl)-L-cysteine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 4099-35-8 | Molecular Weight | 200.68700 | |
| Density | 1.289g/cm3 | Boiling Point | 341.1ºC at 760 mmHg | |
| Molecular Formula | C5H13ClN2O2S | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 160.1ºC | |
Use of S-(2-Aminoethyl)-L-cysteine hydrochlorideThialysine (hydrochloride) is a cysteine derivative[1]. |
| Name | s-(2-aminoethyl)-l-cysteine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Thialysine (hydrochloride) is a cysteine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.289g/cm3 |
|---|---|
| Boiling Point | 341.1ºC at 760 mmHg |
| Molecular Formula | C5H13ClN2O2S |
| Molecular Weight | 200.68700 |
| Flash Point | 160.1ºC |
| Exact Mass | 200.03900 |
| PSA | 114.64000 |
| LogP | 1.29280 |
| Index of Refraction | 1.57 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | HA1690000 |
| HS Code | 2930909090 |
|
~82% 
S-(2-Aminoethyl... CAS#:4099-35-8 |
| Literature: Arnold,L.D.; May,R.G.; Vederas,J.C. Journal of the American Chemical Society, 1988 , vol. 110, p. 2237 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Evidence for conformational movement and radical mechanism in the reaction of 4-thia-L-lysine with lysine 5,6-aminomutase.
J. Phys. Chem. B 113(36) , 12161-3, (2009) We demonstrate that the steady state reaction of lysine 5,6-aminomutase with substrate analogue 4-thia-l-lysine generates a radical intermediate, which accumulates in the enzyme to an electron paramag... |
|
|
Radical triplets and suicide inhibition in reactions of 4-thia-D- and 4-thia-L-lysine with lysine 5,6-aminomutase.
Biochemistry 48(34) , 8151-60, (2009) Lysine 5,6-aminomutase (5,6-LAM) catalyzes the interconversions of D- or L-lysine and the corresponding enantiomers of 2,5-diaminohexanoate, as well as the interconversion of L-beta-lysine and l-3,5-d... |
|
|
Inhibition of lysine 2,3-aminomutase by the alternative substrate 4-thialysine and characterization of the 4-thialysyl radical intermediate.
Arch. Biochem. Biophys. 387(2) , 281-8, (2001) Lysine 2,3-aminomutase catalyzes the interconversion of L-lysine and L-beta-lysine. 4-Thia-L-lysine (4-thialysine) is an alternative substrate for Lysine 2,3-aminomutase. The organic free radical that... |
| H-Cys(aminoethyl)-OH |
| H-CYS(ET-NH2)-OH HCL |
| S-(2-aminoethyl)-L-cysteine monohydrochloride |
| CYSTEINE(AMINOETHYL)-OH HCL |
| L-4-thialysine hydrochloride |
| MFCD00036385 |
| usafxr-43 |
| EINECS 223-862-1 |
| S-(2-Amino-aethyl)-L-cystein,Hydrochlorid |
| S-(aminoethyl)-L-cysteine hydrochloride |
| thialysinehydrochloride |
| S-(2-amino-ethyl)-L-cysteine,hydrochloride |
| lj226 |
| L-Thialysin Hydrochlorid |
| H-CYS(AMINOETHYL)-OH HCL |
| THIALSINE |